The role of P21-activated kinase (Pak1) in sinus node function
- PMID: 37086972
- PMCID: PMC10294268
- DOI: 10.1016/j.yjmcc.2023.04.004
The role of P21-activated kinase (Pak1) in sinus node function
Abstract
Sinoatrial node (SAN) dysfunction (SND) and atrial arrhythmia frequently occur simultaneously with a hazard ratio of 4.2 for new onset atrial fibrillation (AF) in SND patients. In the atrial muscle attenuated activity of p21-activated kinase 1 (Pak1) increases the risk for AF by enhancing NADPH oxidase 2 dependent production of reactive oxygen species (ROS). However, the role of Pak1 dependent ROS regulation in SAN function has not yet been determined. We hypothesize that Pak1 activity maintains SAN activity by regulating the expression of the hyperpolarization activated cyclic nucleotide gated cation channel (HCN). To determine Pak1 dependent changes in heart rate (HR) regulation we quantified the intrinsic sinus rhythm in wild type (WT) and Pak1 deficient (Pak1-/-) mice of both sexes in vivo and in isolated Langendorff perfused hearts. Pak1-/- hearts displayed an attenuated HR in vivo after autonomic blockage and in isolated hearts. The contribution of the Ca2+ clock to pacemaker activity remained unchanged, but Ivabradine (3 μM), a blocker of HCN channels that are a membrane clock component, eliminated the differences in SAN activity between WT and Pak1-/- hearts. Reduced HCN4 expression was confirmed in Pak1-/- right atria. The reduced HCN activity in Pak1-/- could be rescued by class II HDAC inhibition (LMK235), ROS scavenging (TEMPOL) or attenuation of Extracellular Signal-Regulated Kinase (ERK) 1/2 activity (SCH772984). No sex specific differences in Pak1 dependent SAN regulation were determined. Our results establish Pak1 as a class II HDAC regulator and a potential therapeutic target to attenuate SAN bradycardia and AF susceptibility.
Keywords: Atrial fibrillation; HCN channel; Membrane clock; Reactive oxygen species; Sinoatrial node; p21-activated kinase 1.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interests:
The authors have no competing interests.
Figures
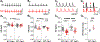



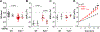

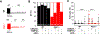
References
-
- Egom EE, Vella K, Hua R, Jansen HJ, Moghtadaei M, Polina I, Bogachev O, Hurnik R, Mackasey M, Rafferty S, Ray G, Rose RA, Impaired sinoatrial node function and increased susceptibility to atrial fibrillation in mice lacking natriuretic peptide receptor C., J. Physiol. (Lond.). 593 (2015) 1127–1146. 10.1113/jphysiol.2014.283135. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

