Asking informed consent may lead to significant participation bias and suboptimal cardiovascular risk management in learning healthcare systems
- PMID: 37087415
- PMCID: PMC10122202
- DOI: 10.1186/s12874-023-01924-6
Asking informed consent may lead to significant participation bias and suboptimal cardiovascular risk management in learning healthcare systems
Abstract
Background: The Utrecht Cardiovascular Cohort - CardioVascular Risk Management (UCC-CVRM) was set up as a learning healthcare system (LHS), aiming at guideline based cardiovascular risk factor measurement in all patients in routine clinical care. However, not all patients provided informed consent, which may lead to participation bias. We aimed to study participation bias in a LHS by assessing differences in and completeness of cardiovascular risk management (CVRM) indicators in electronic health records (EHRs) of consenting, non-consenting, and non-responding patients, using the UCC-CVRM as an example.
Methods: All patients visiting the University Medical Center Utrecht for first time evaluation of a(n) (a)symptomatic vascular disease or condition were invited to participate. Routine care data was collected in the EHR and an informed consent was asked. Differences in patient characteristics were compared between consent groups. We performed multivariable logistic regression to identify determinants of non-consent. We used multinomial regression for an exploratory analysis for the determinants of non-response. Presence of CVRM indicators were compared between consent groups. A waiver (19/641) was obtained from our ethics committee.
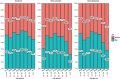
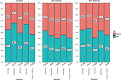
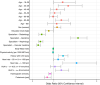
Results: Out of 5730 patients invited, 2378 were consenting, 1907 non-consenting, and 1445 non-responding. Non-consent was related to young and old age, lower education level, lower BMI, physical activity and haemoglobin levels, higher heartrate, cardiovascular disease history and absence of proteinuria. Non-response increased with young and old age, higher education level, physical activity, HbA1c and decreased with lower levels of haemoglobin, BMI, and systolic blood pressure. Presence of CVRM indicators was 5-30% lower in non-consenting patients and even lower in non-responding patients, compared to consenting patients. Non-consent and non-response varied across specialisms.
Conclusions: A traditional informed consent procedure in a LHS may lead to participation bias and potentially to suboptimal CVRM, which is detrimental for feedback on findings in a LHS. This underlines the importance of reassessing the informed consent procedure in a LHS.
Keywords: CVRM; Cardiovascular diseases; Informed consent; Learning healthcare system; Participation bias.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Comparison of the Response to an Electronic Versus a Traditional Informed Consent Procedure in Terms of Clinical Patient Characteristics: Observational Study.J Med Internet Res. 2024 Jul 11;26:e54867. doi: 10.2196/54867. J Med Internet Res. 2024. PMID: 38990640 Free PMC article.
-
Optimizing cardiovascular risk assessment and registration in a developing cardiovascular learning health care system: Women benefit most.PLOS Digit Health. 2023 Feb 8;2(2):e0000190. doi: 10.1371/journal.pdig.0000190. eCollection 2023 Feb. PLOS Digit Health. 2023. PMID: 36812613 Free PMC article.
-
Evaluating a cardiovascular disease risk management care continuum within a learning healthcare system: a prospective cohort study.BJGP Open. 2020 Dec 15;4(5):bjgpopen20X101109. doi: 10.3399/bjgpopen20X101109. Print 2020 Dec. BJGP Open. 2020. PMID: 33144367 Free PMC article.
-
The effect of computerized decision support systems on cardiovascular risk factors: a systematic review and meta-analysis.BMC Med Inform Decis Mak. 2019 Jun 10;19(1):108. doi: 10.1186/s12911-019-0824-x. BMC Med Inform Decis Mak. 2019. PMID: 31182084 Free PMC article.
-
Opt-In and Opt-Out Consent Procedures for the Reuse of Routinely Recorded Health Data in Scientific Research and Their Consequences for Consent Rate and Consent Bias: Systematic Review.J Med Internet Res. 2023 Feb 28;25:e42131. doi: 10.2196/42131. J Med Internet Res. 2023. PMID: 36853745 Free PMC article.
Cited by
-
Comparison of the Response to an Electronic Versus a Traditional Informed Consent Procedure in Terms of Clinical Patient Characteristics: Observational Study.J Med Internet Res. 2024 Jul 11;26:e54867. doi: 10.2196/54867. J Med Internet Res. 2024. PMID: 38990640 Free PMC article.
-
Uncovering variation in cholecystitis treatment: protocol and statistical analysis plan for a nationwide observational study - the Dutch Cholecystitis Snapshot Study (Dutch CHESS).BMJ Open. 2025 May 13;15(5):e093821. doi: 10.1136/bmjopen-2024-093821. BMJ Open. 2025. PMID: 40360397 Free PMC article.
-
Bias in obtaining broad consent in a German general practice? - Preliminary results from a cross-sectional study.J Family Med Prim Care. 2024 Sep;13(9):4056-4065. doi: 10.4103/jfmpc.jfmpc_1957_23. Epub 2024 Sep 11. J Family Med Prim Care. 2024. PMID: 39464962 Free PMC article.
References
-
- Wiersma T, Smulders YM, Stehouwer CDA, Konings KTS, Lanphen J. Summary of the multidisciplinary guideline on cardiovascular risk management (revision 2011) Nederlands tijdschrift voor geneeskunde. 2012;156(36):A5104. - PubMed
-
- WHO . HEARTS technical package for cardiovascular disease management in primary health care: risk based CVD management. Geneva: World Health Organization; 2020. pp. 10–74.
-
- Kotseva K, Wood D, De Bacquer D, De Backer G, Rydén L, Jennings C, et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiolog. 2016;23(6):636–48. doi: 10.1177/2047487315569401. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

