Development of an endoplasmic reticulum stress-related signature with potential implications in prognosis and immunotherapy in head and neck squamous cell carcinoma
- PMID: 37087456
- PMCID: PMC10122290
- DOI: 10.1186/s13000-023-01338-4
Development of an endoplasmic reticulum stress-related signature with potential implications in prognosis and immunotherapy in head and neck squamous cell carcinoma
Abstract
Background: Head and neck squamous cell carcinoma (HNSCC) is a multisite malignancy that responds well to immunotherapy. Despite the initial enthusiasm, the clinical benefits of immunotherapy in HNSCC patients are overall limited. Endoplasmic reticulum stress (ERS) has been indicated to play a key role in the process of anti-tumor immune response mediation. However, ERS-related biomarkers which can accurately predict prognosis and immunotherapy response in HNSCC are still lacking.
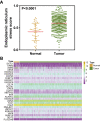
Methods and results: In this study, we identify and validate an ERS-related signature comprises of six genes (ASNS, EXOSC6, BAK1, TPP1, EXOSC8, and TATDN2) that can predict the prognosis of HNSCC patients. GSEA analysis indicates that the ERS-related signature is significantly correlated with tumor immunity in HNSCC. Moreover, the infiltration of naive B cells and CD8 + T cells are significantly diminished in patients with high-risk scores compared to those with low-risk scores, while macrophages and activated mast cells are remarkably enhanced. Furthermore, the ERS-related signature also displays a tremendous potential for predicting immunotherapy response in HNSCC.
Conclusions: Our study identifies an ERS-related signature that can predict the prognosis of HNSCC patients and highlights its potential value as a predictive biomarker of immunotherapy response, potentially enabling more precise and personalized immunotherapy response and paving the way for further investigation of the prognostic and therapeutic potentials of ERS.
Keywords: Biomarker; Endoplasmic reticulum stress; Head and neck squamous cell carcinoma; Immunotherapy; Prognosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Construction and validation of a prognostic signature based on seven endoplasmic reticulum stress-related lncRNAs for patients with head and neck squamous cell carcinoma.Sci Rep. 2023 Dec 16;13(1):22414. doi: 10.1038/s41598-023-49987-1. Sci Rep. 2023. PMID: 38104177 Free PMC article.
-
Comprehensive analysis of endoplasmic reticulum stress related signature in head and neck squamous carcinoma.Sci Rep. 2024 Jul 23;14(1):16972. doi: 10.1038/s41598-024-65090-5. Sci Rep. 2024. PMID: 39043683 Free PMC article.
-
Mast cell marker gene signature in head and neck squamous cell carcinoma.BMC Cancer. 2022 May 24;22(1):577. doi: 10.1186/s12885-022-09673-3. BMC Cancer. 2022. PMID: 35610596 Free PMC article.
-
Advances in tumor immune microenvironment of head and neck squamous cell carcinoma: A review of literature.Medicine (Baltimore). 2024 Mar 1;103(9):e37387. doi: 10.1097/MD.0000000000037387. Medicine (Baltimore). 2024. PMID: 38428879 Free PMC article. Review.
-
Immunotherapy in head and neck squamous cell carcinoma: An updated review.Cancer Treat Res Commun. 2022;33:100649. doi: 10.1016/j.ctarc.2022.100649. Epub 2022 Oct 14. Cancer Treat Res Commun. 2022. PMID: 36279709 Review.
Cited by
-
A novel mast cell marker gene-related prognostic signature to predict prognosis and reveal the immune landscape in head and neck squamous cell carcinoma.Front Immunol. 2025 Jul 9;16:1538641. doi: 10.3389/fimmu.2025.1538641. eCollection 2025. Front Immunol. 2025. PMID: 40703522 Free PMC article.
-
The Complex Role of Mast Cells in Head and Neck Squamous Cell Carcinoma: A Systematic Review.Medicina (Kaunas). 2024 Jul 19;60(7):1173. doi: 10.3390/medicina60071173. Medicina (Kaunas). 2024. PMID: 39064602 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

