NO-mediated dormancy release of Avena fatua caryopses is associated with decrease in abscisic acid sensitivity, content and ABA/GAs ratios
- PMID: 37087501
- PMCID: PMC10122620
- DOI: 10.1007/s00425-023-04117-z
NO-mediated dormancy release of Avena fatua caryopses is associated with decrease in abscisic acid sensitivity, content and ABA/GAs ratios
Abstract
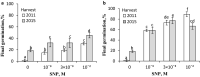
NO releases caryopsis dormancy in Avena fatua, the effect being dependent on the level of dormancy. The NO effect involves also the reduction of caryopsis sensitivity to ABA and to a decrease in the ABA to GAs ratio due to a decrease in ABA levels and the lack of effect on GAs levels before germination is completed. Nitric oxide (NO) from various donors (i.e. SNP, GSNO and acidified KNO2), applied to dry caryopses or during initial germination, released primary dormancy in caryopses. Dormancy in caryopses was gradually lost during dry storage (after-ripening) at 25 °C, enabling germination at 20 °C in the dark. The after-ripening effect is associated with a decrease in NO required for germination. In addition, NO decreased the sensitivity of dormant caryopses to exogenous abscisic acid (ABA) and decreased the embryos' ABA content before germination was completed. However, NO did not affect the content of bioactive gibberellins (GAs) from non-13-hydroxylation (GA4, GA7) and 13-hydroxylation (GA1, GA3, GA6.) pathways. Paclobutrazol (PAC), commonly regarded as a GAs biosynthesis inhibitor, counteracted the dormancy-releasing effect of NO and did not affect the GAs level; however, it increased the ABA content in embryos before germination was completed. Ascorbic acid, sodium benzoate and tiron, scavengers of reactive oxygen species (ROS), reduced the stimulatory effect of NO on caryopsis germination. This work provides new insight on the participation of NO in releasing A. fatua caryopses dormancy and on the relationship of NO with endogenous ABA and GAs.
Keywords: Abscisic acid; After ripening; Avena fatua; Dormancy; Gibberellins; Nitric oxide.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
KAR1-induced dormancy release in Avena fatua caryopses involves reduction of caryopsis sensitivity to ABA and ABA/GAs ratio in coleorhiza and radicle.Planta. 2024 Apr 18;259(6):126. doi: 10.1007/s00425-024-04387-1. Planta. 2024. PMID: 38635035 Free PMC article.
-
After-Ripening Is Associated with Changes in the Sensitivity of Avena fatua L. Caryopses to Abscisic Acid, as Well as Changes in the Abscisic Acid and Bioactive Gibberellins Contents in Embryos.Plants (Basel). 2025 Feb 5;14(3):463. doi: 10.3390/plants14030463. Plants (Basel). 2025. PMID: 39943025 Free PMC article.
-
Gibberellin-like effects of KAR1 on dormancy release of Avena fatua caryopses include participation of non-enzymatic antioxidants and cell cycle activation in embryos.Planta. 2016 Feb;243(2):531-48. doi: 10.1007/s00425-015-2422-1. Epub 2015 Nov 2. Planta. 2016. PMID: 26526413 Free PMC article.
-
The role of nitric oxide in the germination of plant seeds and pollen.Plant Sci. 2011 Nov;181(5):560-72. doi: 10.1016/j.plantsci.2011.03.014. Epub 2011 Apr 6. Plant Sci. 2011. PMID: 21893253 Review.
-
The roles of auxin in seed dormancy and germination.Yi Chuan. 2016 Apr;38(4):314-22. doi: 10.16288/j.yczz.15-464. Yi Chuan. 2016. PMID: 27103455 Review.
Cited by
-
KAR1-induced dormancy release in Avena fatua caryopses involves reduction of caryopsis sensitivity to ABA and ABA/GAs ratio in coleorhiza and radicle.Planta. 2024 Apr 18;259(6):126. doi: 10.1007/s00425-024-04387-1. Planta. 2024. PMID: 38635035 Free PMC article.
-
A Travel through Landscapes of Seed Dormancy.Plants (Basel). 2023 Nov 24;12(23):3963. doi: 10.3390/plants12233963. Plants (Basel). 2023. PMID: 38068600 Free PMC article. Review.
-
After-Ripening Is Associated with Changes in the Sensitivity of Avena fatua L. Caryopses to Abscisic Acid, as Well as Changes in the Abscisic Acid and Bioactive Gibberellins Contents in Embryos.Plants (Basel). 2025 Feb 5;14(3):463. doi: 10.3390/plants14030463. Plants (Basel). 2025. PMID: 39943025 Free PMC article.
References
-
- Amory AM, Ford L, Pammenter NW, Cressswell CF. The use of 3-amino-1.2,4-triazole to investigate the short –term effects of oxygen toxicity on carbon assimilation by Pisum sativum seedlings. Plant Cell Environ. 1992;15:655–663. doi: 10.1111/j.1365-3040.1992.tb01007.x. - DOI
-
- Andryka-Dudek P, Ciacka K, Wiśniewska A, Bogatek R, Gniazdowska A. Nitric oxide-induced dormancy removal of apple embryos is linked to alterations in expression of genes encoding ABA and JA biosynthetic or transduction pathways and RNA nitration. Int J Mol Sci. 2019;20:2–17. doi: 10.3390/ijms20051007. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

