Simple and safe digitoxin dosing in heart failure based on data from the DIGIT-HF trial
- PMID: 37087503
- PMCID: PMC10359203
- DOI: 10.1007/s00392-023-02199-z
Simple and safe digitoxin dosing in heart failure based on data from the DIGIT-HF trial
Abstract
Background: The present study aimed to develop a simple dosing score when starting the cardiac glycoside digitoxin in heart failure with reduced ejection fraction (HFrEF) employing first data from the randomized, double-blinded DIGIT-HF trial.
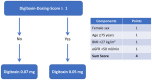
Methods and results: In DIGIT-HF, digitoxin was started with a dose of 0.07 mg once daily (o.d.) in all patients. For score derivation, 317 patients were analyzed who had been randomized to digitoxin. In these patients, after scheduled determination of serum levels at study week 6, the digitoxin dose had remained unchanged or had been reduced to 0.05 mg o.d. (97% of patients) to achieve serum concentrations within a predefined range (10.5-23.6 nmol/l). In logistic regression analyses, sex, age, body mass index (BMI), and estimated glomerular filtration rate (eGFR) were associated with need for dose reduction and, therefore, selected for further developing the dosing score. Optimal cut-points were derived from ROC curve analyses. Finally, female sex, age ≥ 75 years, eGFR < 50 ml/min/1.73 m2, and BMI < 27 kg/m2 each were assigned one point for the digitoxin dosing score. A score of ≥ 1 indicated the need for dose reduction with sensitivity/specificity of 81.6%/49.7%, respectively. Accuracy was confirmed in a validation data set including 64 patients randomized to digitoxin yielding sensitivity/specificity of 87.5%/37.5%, respectively.
Conclusion: In patients with HFrEF, treatment with digitoxin should be started at 0.05 mg o.d. in subjects with either female sex, eGFR < 50 ml/min/1.73m2, BMI < 27 kg/m2, or age ≥ 75 years. In any other patient, digitoxin may be safely started at 0.07 mg o.d.
Keywords: Cardiac glycosides; Clinical trial; Digitoxin; Dose titration; Heart failure.
© 2023. The Author(s).
Conflict of interest statement
U.B. and J.B. represent the study heads of the DIGIT-HF-study and applied for funding of DIGIT-HF described above. U.B., J.B., A.K. H. v. d. L., C. V., M. B. and S.S. are members of the DIGIT-HF trial steering committee. U.B. received travel support and honoraria for lectures/consulting from Alnylam Pharmaceutical, Amgen, Astra Zeneca, Bayer Vital, Novartis, and Pfizer and institutional research support from Alnylam Pharmaceuticals, all not related to the current manuscript. J.B. has received honoraria for lectures/consulting from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor and institutional research support from Zoll, CVRx, Abiomed, all not related to the current manuscript. MB is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322900939) and reports personal fees from Abbott, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Edwards, Medtronic, Novartis, ReCor, Servier and Vifor during the conduct of the study. A.J.R. received honoraria for lectures from AstraZeneca and Bayer, and travel support from Johnson&Johnson and Servier, all not related to the current manuscript. C.V. received honoraria for lectures/consulting from Abbott, Bayer, Biotronik, BMS, Boehringer Ingelheim, CVRx and Medtronic, all not related to the current manuscript. H.v.L is serving as medical director of Orgenesis, Inc, unrelated to the current manuscript. L.S.M. has no conflicts of interest with respect to the drug studied in this trial. MH received speakerhonoraria, grants and advisory honoraria form Roche Diagnostics, Boehringer Ingelheim, Astra Zeneca, Novartis, Vifor, Biopeutics, Sandoz, Baxter. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. - DOI - PubMed
-
- The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med 1997;336:525–533. doi: 10.1056/NEJM199702203360801 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

