UTP11 deficiency suppresses cancer development via nucleolar stress and ferroptosis
- PMID: 37087976
- PMCID: PMC10149416
- DOI: 10.1016/j.redox.2023.102705
UTP11 deficiency suppresses cancer development via nucleolar stress and ferroptosis
Abstract
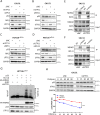
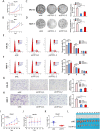
The eukaryotic ribosome is essential for cancer cell survival. Perturbation of ribosome biogenesis induces nucleolar stress or ribosomal stress, which restrains cancer growth, as rapidly proliferating cancer cells need more active ribosome biogenesis. In this study, we found that UTP11 plays an important role in the biosynthesis of 18S ribosomal RNAs (rRNA) by binding to the pre-rRNA processing factor, MPP10. UTP11 is overexpressed in human cancers and associated with poor prognoses. Interestingly, depletion of UTP11 inhibits cancer cell growth in vitro and in vivo through p53-depedednt and -independent mechanisms, whereas UTP11 overexpression promotes cancer cell growth and progression. On the one hand, the ablation of UTP11 impedes 18S rRNA biosynthesis to trigger nucleolar stress, thereby preventing MDM2-mediated p53 ubiquitination and degradation through ribosomal proteins, RPL5 and RPL11. On the other hand, UTP11 deficiency represses the expression of SLC7A11 by promoting the decay of NRF2 mRNA, resulting in reduced levels of glutathione (GSH) and enhanced ferroptosis. Altogether, our study uncovers a critical role for UTP11 in maintaining cancer cell survival and growth, as depleting UTP11 leads to p53-dependent cancer cell growth arrest and p53-independent ferroptosis.
Keywords: Ferroptosis; Nucleolar stress; Ribosome biogenesis; SLC7A11; UTP11; p53.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures





References
-
- Pelletier J., Thomas G., Volarevic S. Ribosome biogenesis in cancer: new players and therapeutic avenues. Nat. Rev. Cancer. 2018;18(1):51–63. - PubMed
-
- Zhou X., Lu H. In: Encyclopedia of Cell Biology. Bradshaw R., Hart G., Stahl P., editors. Academic Press; 2023. Extra-ribosome functions of ribosomal proteins; pp. 57–70.
-
- van Riggelen J., Yetil A., Felsher D.W. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat. Rev. Cancer. 2010;10(4):301–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

