Ultrasensitive Protein Detection Technologies for Extracellular Vesicle Measurements
- PMID: 37088150
- PMCID: PMC10326690
- DOI: 10.1016/j.mcpro.2023.100557
Ultrasensitive Protein Detection Technologies for Extracellular Vesicle Measurements
Abstract
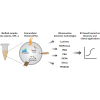
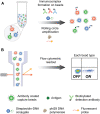
Extracellular vesicles (EVs) are nanoscopic, heterogenous, lipid-rich particles that carry a multitude of cargo biomolecules including proteins, nucleic acids, and metabolites. Although historically EVs were regarded as cellular debris with no intrinsic value, growing understanding of EV biogenesis has led to the realization that EVs facilitate intercellular communication and are sources of liquid biomarkers. EVs can be isolated and analyzed from a wide variety of accessible biofluids for biomarker discovery and diagnostic applications. There is a diversity of EVs from different biological compartments (e.g., cells and tissues), and some of these EVs are present at extremely low concentrations. Consequently, a challenge in the field is to find appropriate markers that enable selective isolation of these rare EVs. Many conventional protein detection technologies have limited sensitivity to detect low abundance biomarkers in EVs, limiting their use in EV research. Advances in ultrasensitive detection technologies are needed to harness the potential of EVs for clinical application. This Perspective highlights current EV research focusing on ultrasensitive detection technologies, their limitations, and areas of potential growth in the future.
Keywords: biofluids; biomarkers; extracellular vesicles; proteins; ultrasensitive technology.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: A. S. and M. N. declare no known competing financial interests. D. R. W. has a financial interest in Quanterix, a company developing an ultrasensitive digital immunoassay platform; he is an inventor of the Simoa technology, a founder of the company, and a member of its board of directors. D. R. W.’s interests were reviewed and are managed by BWH. and Partners HealthCare in accordance with their conflict-of-interest policies.
Figures

References
-
- Wolf P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967;13:269–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

