Meta-analysis: Prevalence of significant or advanced fibrosis in adults with alpha-1-antitrypsin deficiency
- PMID: 37089038
- PMCID: PMC10330074
- DOI: 10.1111/apt.17516
Meta-analysis: Prevalence of significant or advanced fibrosis in adults with alpha-1-antitrypsin deficiency
Abstract
Background: The prevalence of liver fibrosis detected by non-invasive imaging in alpha-1-antitrypsin (AAT) deficiency has not been systematically assessed.
Aims: We conducted a systematic review and meta-analysis to determine the prevalence of significant fibrosis and advanced fibrosis in AAT deficiency based on non-invasive imaging.
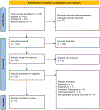
Methods: Medline and Embase electronic databases were searched for studies from inception to 13 November 2022 that provided data for the prevalence of fibrosis in adults with AAT deficiency. A generalised linear mixed model with Clopper-Pearson intervals was used to pool single-arm outcomes.
Results: Of the 214 records identified, 8 studies were included. Five studies assessed fibrosis using vibration-controlled transient elastography. The prevalence of significant fibrosis (defined as ≥7.1 kPA) in Z homozygosity, Z heterozygosity and non-carrier status was 22.10% (five studies, 95% CI: 17.07-28.12), 9.24% (three studies, 95% CI: 4.68-17.45) and 5.38% (one study, 95% CI: 3.27-8.73), respectively, p < 0.0001, and the prevalence of advanced fibrosis (defined as ≥9.5 kPa) was 8.13% (five studies, 95% CI: 4.60-13.96), 2.96% (three studies, 95% CI: 1.49-5.81) and 1.08% (one study, 95% CI: 0.35-3.28), respectively, p = 0.003. There were limited data regarding the use of magnetic resonance elastography or acoustic radiation force impulse to assess for fibrosis.
Conclusion: More than one in five adult individuals with AAT deficiency and Z homozygosity harbour significant fibrosis, and nearly 1 in 10 harbours advanced fibrosis. The risk of fibrosis increases incrementally with the frequency of Pi*Z mutations.
© 2023 John Wiley & Sons Ltd.
Conflict of interest statement
R.L. serves as a consultant or advisory board member for Anylam/Regeneron, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Inipharm, Intercept, Ionis, Janssen, Merck, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Promethera, Sagimet, 89bio, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer, pH Pharma, and Siemens. He is also co-founder of Liponexus. D.H serves as an advisory board member for Eisai. P.M serves as a consultant of advisory member for Ipsen, Eisai, Abbvie, Sanofi, Gilead Sciences, Evive Biotech, Novo Nordisk, Bayer Healthcare, Intercept, Surrozen, and Pfizer. H.C.P lectures and receives advisory board fees from Intercept, Genfit, Promethera Bioscience, Orphalan, Novo Nordisk and Roche Portugal.
Figures


References
-
- Strnad P, McElvaney NG, Lomas DA. Alpha(1)-Antitrypsin Deficiency. N Engl J Med. 2020;382(15):1443–1455. - PubMed
-
- Townsend SA, Edgar RG, Ellis PR, Kantas D, Newsome PN, Turner AM. Systematic review: the natural history of alpha-1 antitrypsin deficiency, and associated liver disease. Aliment Pharmacol Ther. 2018;47(7):877–885. - PubMed
-
- Bowlus CL, Willner I, Zern MA, et al. Factors associated with advanced liver disease in adults with alpha1-antitrypsin deficiency. Clin Gastroenterol Hepatol. 2005;3(4):390–396. - PubMed
-
- Strnad P, Buch S, Hamesch K, et al. Heterozygous carriage of the alpha1-antitrypsin Pi*Z variant increases the risk to develop liver cirrhosis. Gut. 2019;68(6):1099–1107. - PubMed
Publication types
MeSH terms
Grants and funding
- 5UL1TR001442/TR/NCATS NIH HHS/United States
- R01DK121378/DK/NIDDK NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R01DK124318/DK/NIDDK NIH HHS/United States
- P30 DK120515/DK/NIDDK NIH HHS/United States
- U01 AA029019/AA/NIAAA NIH HHS/United States
- P30DK120515/DK/NIDDK NIH HHS/United States
- P01HL147835/HB/NHLBI NIH HHS/United States
- P01 HL147835/HL/NHLBI NIH HHS/United States
- R01 DK121378/DK/NIDDK NIH HHS/United States
- R01 DK106419/DK/NIDDK NIH HHS/United States
- 5P42ES010337/ES/NIEHS NIH HHS/United States
- U01DK061734/DK/NIDDK NIH HHS/United States
- U01 DK130190/DK/NIDDK NIH HHS/United States
- U01DK130190/DK/NIDDK NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- R01 AA028550/AA/NIAAA NIH HHS/United States
- R01 DK124318/DK/NIDDK NIH HHS/United States
- R01DK106419/DK/NIDDK NIH HHS/United States
- U01AA029019/AA/NIAAA NIH HHS/United States
- U01 DK061734/DK/NIDDK NIH HHS/United States
- U01 AA022614/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
