The origin of overpotential in lithium-mediated nitrogen reduction
- PMID: 37089070
- PMCID: PMC10354832
- DOI: 10.1039/d2fd00156j
The origin of overpotential in lithium-mediated nitrogen reduction
Abstract
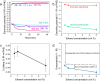
The verification of the lithium-mediated nitrogen reduction system in 2019 has led to an explosion in the literature focussing on improving the metrics of faradaic efficiency, stability, and activity. However, while the literature acknowledges the vast intrinsic overpotential for nitrogen reduction due to the reliance on in situ lithium plating, it has thus far been difficult to accurately quantify this overpotential and effectively analyse further voltage losses. In this work, we present a simple method for determining the Reversible Hydrogen Electrode (RHE) potential in the lithium-mediated nitrogen reduction system. This method allows for an investigation of the Nernst equation and reveals sources of potential losses. These are namely the solvation of the lithium ion in the electrolyte and resistive losses due to the formation of the solid electrolyte interphase. The minimum observed overpotential was achieved in a 0.6 M LiClO4, 0.5 vol% ethanol in tetrahydrofuran electrolyte. This was -3.59 ± 0.07 V vs. RHE, with a measured faradaic efficiency of 6.5 ± 0.2%. Our method allows for easy comparison between the lithium-mediated system and other nitrogen reduction paradigms, including biological and homogeneous mechanisms.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Smith C. Hill A. K. Torrente-Murciano L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020;13:331–344. doi: 10.1039/C9EE02873K. - DOI
-
- Lazouski N. Schiffer Z. J. Williams K. et al., Understanding Continuous Lithium-Mediated Electrochemical Nitrogen Reduction. Joule. 2019;3:1127–1139. doi: 10.1016/j.joule.2019.02.003. - DOI
-
- Martín A. J. Shinagawa T. Pérez-Ramírez J. Electrocatalytic Reduction of Nitrogen: From Haber-Bosch to Ammonia Artificial Leaf. Chem. 2019;5:263–283.
-
- Tsuneto A. Kudo A. Sakata T. Lithium-mediated electrochemical reduction of high pressure N2 to NH3. J. Electroanal. Chem. 1994;367:183–188. doi: 10.1016/0022-0728(93)03025-K. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

