Hyperpolarization electrical signals induced by local action of moderate heating influence photosynthetic light reactions in wheat plants
- PMID: 37089652
- PMCID: PMC10113467
- DOI: 10.3389/fpls.2023.1153731
Hyperpolarization electrical signals induced by local action of moderate heating influence photosynthetic light reactions in wheat plants
Abstract
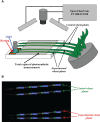
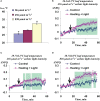
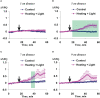
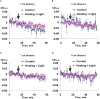
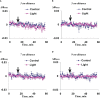
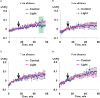
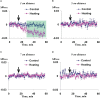
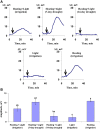
Local action of stressors induces fast changes in physiological processes in intact parts of plants including photosynthetic inactivation. This response is mediated by generation and propagation of depolarization electrical signals (action potentials and variation potentials) and participates in increasing plant tolerance to action of adverse factors. Earlier, we showed that a local action of physiological stimuli (moderate heating and blue light), which can be observed under environmental conditions, induces hyperpolarization electrical signals (system potentials) in wheat plants. It potentially means that these signals can play a key role in induction of fast physiological changes under the local action of environmental stressors. The current work was devoted to investigation of influence of hyperpolarization electrical signals induced by the local action of the moderate heating and blue light on parameters of photosynthetic light reactions. A quantum yield of photosystem II (ФPSII) and a non-photochemical quenching of chlorophyll fluorescence (NPQ) in wheat plants were investigated. It was shown that combination of the moderate heating (40°C) and blue light (540 µmol m-2s-1) decreased ФPSII and increased NPQ; these changes were observed in 3-5 cm from border of the irritated zone and dependent on intensity of actinic light. The moderate soil drought (7 days) increased magnitude of photosynthetic changes and shifted their localization which were observed on 5-7 cm from the irritated zone; in contrast, the strong soil drought (14 days) suppressed these changes. The local moderate heating decreased ФPSII and increased NPQ without action of the blue light; in contrast, the local blue light action without heating weakly influenced these parameters. It meant that just local heating was mechanism of induction of the photosynthetic changes. Finally, propagation of hyperpolarization electrical signals (system potentials) was necessary for decreasing ФPSII and increasing NPQ. Thus, our results show that hyperpolarization electrical signals induced by the local action of the moderate heating inactivates photosynthetic light reactions; this response is similar with photosynthetic changes induced by depolarization electrical signals. The soil drought and actinic light intensity can influence parameters of these photosynthetic changes.
Keywords: hyperpolarization electrical signals; light; local moderate heating; non-photochemical quenching; photosynthetic response; quantum yield of photosystem II; soil drought.
Copyright © 2023 Yudina, Sukhova, Popova, Zolin, Abasheva, Grebneva and Sukhov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources

