Systemic LRG1 Expression in Melanoma is Associated with Disease Progression and Recurrence
- PMID: 37089863
- PMCID: PMC10117404
- DOI: 10.1158/2767-9764.CRC-23-0015
Systemic LRG1 Expression in Melanoma is Associated with Disease Progression and Recurrence
Abstract
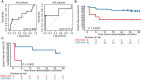
The response rates upon neoadjuvant immune checkpoint blockade (ICB) in stage III melanoma are higher as compared with stage IV disease. Given that successful ICB depends on systemic immune response, we hypothesized that systemic immune suppression might be a mechanism responsible for lower response rates in late-stage disease, and also potentially with disease recurrence in early-stage disease. Plasma and serum samples of cohorts of patients with melanoma were analyzed for circulating proteins using mass spectrometry proteomic profiling and Olink proteomic assay. A cohort of paired samples of patients with stage III that progressed to stage IV disease (n = 64) was used to identify markers associated with higher tumor burden. Baseline patient samples from the OpACIN-neo study (n = 83) and PRADO study (n = 49; NCT02977052) were used as two independent cohorts to analyze whether the potential identified markers are also associated with disease recurrence after neoadjuvant ICB therapy. When comparing baseline proteins overlapping between patients with progressive disease and patients with recurrent disease, we found leucine-rich alpha-2-glycoprotein 1 (LRG1) to be associated with worse prognosis. Especially nonresponder patients to neoadjuvant ICB (OpACIN-neo) with high LRG1 expression had a poor outcome with an estimated 36-month event-free survival of 14% as compared with 83% for nonresponders with a low LRG1 expression (P = 0.014). This finding was validated in an independent cohort (P = 0.0021). LRG1 can be used as a biomarker to identify patients with high risk for disease progression and recurrence, and might be a target to be combined with neoadjuvant ICB.
Significance: LRG1 could serve as a potential target and as a biomarker to identify patients with high risk for disease recurrence, and consequently benefit from additional therapies and intensive follow-up.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures



Similar articles
-
Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma.Nat Med. 2021 Feb;27(2):256-263. doi: 10.1038/s41591-020-01211-7. Epub 2021 Feb 8. Nat Med. 2021. PMID: 33558721 Clinical Trial.
-
Survival update of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma in the OpACIN and OpACIN-neo trials.Ann Oncol. 2023 Apr;34(4):420-430. doi: 10.1016/j.annonc.2023.01.004. Epub 2023 Jan 18. Ann Oncol. 2023. PMID: 36681299 Clinical Trial.
-
Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial.Lancet Oncol. 2019 Jul;20(7):948-960. doi: 10.1016/S1470-2045(19)30151-2. Epub 2019 May 31. Lancet Oncol. 2019. PMID: 31160251 Clinical Trial.
-
Biomarkers of Immune Checkpoint Blockade Response in Triple-Negative Breast Cancer.Curr Treat Options Oncol. 2021 Mar 20;22(5):38. doi: 10.1007/s11864-021-00833-4. Curr Treat Options Oncol. 2021. PMID: 33743085 Review.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
Cited by
-
Plasma Profiling of Acute Myeloid Leukemia With Fever- and Infection-Related Complications During Chemotherapy-Induced Neutropenia.Cancer Rep (Hoboken). 2024 Oct;7(10):e70024. doi: 10.1002/cnr2.70024. Cancer Rep (Hoboken). 2024. PMID: 39441646 Free PMC article.
-
The disruptive role of LRG1 on the vasculature and perivascular microenvironment.Front Cardiovasc Med. 2024 Apr 30;11:1386177. doi: 10.3389/fcvm.2024.1386177. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38745756 Free PMC article. Review.
-
CD39 and LDHA affects the prognostic role of NLR in metastatic melanoma patients treated with immunotherapy.J Transl Med. 2023 Sep 8;21(1):610. doi: 10.1186/s12967-023-04419-6. J Transl Med. 2023. PMID: 37684649 Free PMC article.
-
Angiogenesis and targeted therapy in the tumour microenvironment: From basic to clinical practice.Clin Transl Med. 2025 Apr;15(4):e70313. doi: 10.1002/ctm2.70313. Clin Transl Med. 2025. PMID: 40268524 Free PMC article. Review.
References
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. . Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

