Clinical characteristics and management of immune checkpoint inhibitor-related cardiotoxicity: A single-center experience
- PMID: 37089888
- PMCID: PMC10115988
- DOI: 10.3389/fcvm.2023.1093383
Clinical characteristics and management of immune checkpoint inhibitor-related cardiotoxicity: A single-center experience
Abstract
Background: Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy in the past decade and amplify T-cell-mediated immune responses by disrupting immunoinhibitory signals. The augmented T-cell immune response has led to a range of immune-related adverse effects (irAEs). Immune-related cardiotoxicity has been reported in case series but has been underappreciated due to difficulties in diagnosis. This article describes epidemiological, clinical presentation, subtype, and treatment data and a new systematic framework for the clinical management of cardiotoxicity.
Methods: Data were extracted for cancer patients who received ICIs in a single center between January 1, 2020, and February 28, 2022. ICI-associated cardiotoxicity was clinically diagnosed based on clinical presentations, biochemical biomarkers, and imaging features.
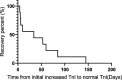
Results: We identified a total of 12 (2.46%) cases of ICI-related cardiotoxicity from 487 patients who received PD-1 or PD-L1 inhibitors. All patients were diagnosed with advanced or metastatic solid tumors. The severity of ICI-related cardiotoxicity ranged from subclinical cardiac abnormalities (subclinical type) with only asymptomatic troponin-I (TnI) elevations (25.0%) to symptomatic cardiac abnormalities (clinical type) (75.0%). Patients with symptomatic cardiac abnormalities had several manifestations, including tachyarrhythmia (16.7%), bradyarrhythmia (41.7%), or cardiac failure (8.3%). The median immunotherapy exposure time was 1.5 doses (range: 1 to 5), and the median time from the initial immunotherapy to the onset of ICI-related cardiotoxicity was 33.5 days (IQR: 20.3 to 46.8). Most patients, including those with subclinical cardiac abnormalities, were administered systemic corticosteroids (58.3%). One (8.3%) patient was put on mechanical ventilation, one (8.3%) received plasma exchange therapy, one (8.3%) was implanted with a pacemaker, and one (8.3%) was admitted to the ICU. Three patients with symptomatic cardiac abnormalities (25.0%) died, and other patients presented with significant clinical improvement with good outcomes.
Conclusion: ICI-related cardiotoxicity is uncommon but critical with a high mortality rate and poor prognosis, especially for a small group of patients with symptomatic cardiac abnormalities. More attention should be given to cardiotoxicity associated with ICIs, and these patients should be given baseline examinations and biochemical analyses before and after the initiation of immunotherapy, intensive cardiac assessments, an accurate and rapid diagnosis, and timely multidisciplinary management with immunosuppressive agents and other necessary clinical interventions.
Keywords: PD-1; PD-L1; cardiotoxicity; immune checkpoint inhibitor; immune-related adverse event; myocarditis.
© 2023 Xiao, Li, Wang, Guan, Liu, Liang, Li, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous