Prescribing Trends for the Same Patients with Schizophrenia Over 20 Years
- PMID: 37089914
- PMCID: PMC10120815
- DOI: 10.2147/NDT.S390482
Prescribing Trends for the Same Patients with Schizophrenia Over 20 Years
Abstract
Background: Recent pharmacoepidemiology data show an increase in the proportion of patients receiving second-generation antipsychotic (SGA) monotherapy, but no studies have analyzed the same patients over a long period of time. Therefore, in this study, we retrospectively evaluated schizophrenia patients with available data for 20 years to determine whether the drug treatments in the same patients have changed in the past 20 years.
Methods: The study began in April 2021 and was conducted in 15 psychiatric hospitals in Japan. Schizophrenia patients treated in the same hospital for 20 years were retrospectively examined for all prescriptions in 2016, 2011, 2006, and 2001 (ie, every 5 years).
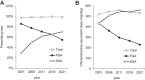
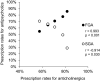
Results: The mean age of the 716 patients surveyed in 2021 was 61.7 years, with 49.0% being female. The rate of antipsychotic monotherapy use showed a slight increasing trend over the past 20 years; the rate of SGA use showed a marked increasing trend from 28.9% to 70.3% over the past 20 years, while the rate of SGA monotherapy use showed a gradual increasing trend over the past 20 years. The rates of concomitant use of anticholinergics, antidepressants, anxiolytics/sleep medications, and mood stabilizers showed decreasing, flat, flat, and flat trends over the past 20 years, respectively.
Conclusion: The results of this study showed a slow but steady substitution of SGAs for first-generation antipsychotics (FGAs) over time, even in the same patients.
Keywords: antipsychotics; pharmacoepidemiology; prescription; schizophrenia; trends.
© 2023 Yasui-Furukori et al.
Conflict of interest statement
Dr. Kensuke Miyazaki reports personal fees from Sumitomo Dainippon Pharma Co., Ltd., outside the submitted work. Professor Kazutaka Shimoda reports grants from Novartis Pharma KK, Dainippon Sumitomo Pharma Co., Astellas Pharma Inc., Meiji Seika Pharma Co., Ltd., Eisai Co., Ltd., Pfizer Inc., Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Takeda Pharmaceutical Co., Ltd.; personal fees from Eisai Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Janssen Pharmaceutical Co., Shionogi & Co. Ltd., Dainippon Sumitomo Pharma Co., Daiichi Sankyo Co., and Pfizer Inc., outside the submitted work. The remaining authors report no other conflicts of interest in this work.
Figures
Similar articles
-
[Prescribing patterns of antipsychotics in 13 French psychiatric hospitals].Encephale. 2009 Apr;35(2):129-38. doi: 10.1016/j.encep.2008.03.007. Epub 2008 Jul 7. Encephale. 2009. PMID: 19393381 French.
-
Effects of first-generation antipsychotics versus second-generation antipsychotics on quality of life in schizophrenia: a double-blind, randomised study.Lancet Psychiatry. 2016 Aug;3(8):717-729. doi: 10.1016/S2215-0366(16)00085-7. Epub 2016 Jun 2. Lancet Psychiatry. 2016. PMID: 27265548 Clinical Trial.
-
Oral antipsychotic prescribing and association with neighbourhood-level socioeconomic status: analysis of time trend of routine primary care data in England, 2011-2016.Soc Psychiatry Psychiatr Epidemiol. 2020 Feb;55(2):165-173. doi: 10.1007/s00127-019-01793-9. Epub 2019 Oct 19. Soc Psychiatry Psychiatr Epidemiol. 2020. PMID: 31630215
-
[Cost-effectiveness analysis of schizophrenic patient care settings: impact of an atypical antipsychotic under long-acting injection formulation].Encephale. 2005 Mar-Apr;31(2):235-46. doi: 10.1016/s0013-7006(05)82390-5. Encephale. 2005. PMID: 15959450 Review. French.
-
Antipsychotics for children and young adults: a comparative effectiveness review.Pediatrics. 2012 Mar;129(3):e771-84. doi: 10.1542/peds.2011-2158. Epub 2012 Feb 20. Pediatrics. 2012. PMID: 22351885 Review.
Cited by
-
Evaluation of Prescription Patterns of Antipsychotics in Schizophrenia Patients-A Single-Center Prospective Study.J Clin Med. 2025 Apr 24;14(9):2941. doi: 10.3390/jcm14092941. J Clin Med. 2025. PMID: 40363973 Free PMC article.
-
Plasmatic Levels and Response to Variable Doses of Monthly Aripiprazole and Three-Month Paliperidone in Patients with Severe Schizophrenia. Treatment Adherence, Effectiveness, Tolerability, and Safety.Neuropsychiatr Dis Treat. 2023 Oct 5;19:2093-2103. doi: 10.2147/NDT.S425516. eCollection 2023. Neuropsychiatr Dis Treat. 2023. PMID: 37818449 Free PMC article.
-
Should we be Prescribing Fluphenazine Long-Acting Injectable Formulation?Curr Psychiatry Rep. 2025 Jun;27(6):408-414. doi: 10.1007/s11920-025-01610-y. Epub 2025 Apr 28. Curr Psychiatry Rep. 2025. PMID: 40289033 Free PMC article. Review.
-
Antipsychotic prescription patterns among schizophrenia patients in Guangdong Province, China's 686 program: A retrospective study.Medicine (Baltimore). 2024 Sep 13;103(37):e39629. doi: 10.1097/MD.0000000000039629. Medicine (Baltimore). 2024. PMID: 39298629 Free PMC article.
-
Factors associated with the initiation of laxative use in the same patients with schizophrenia over a 20-year period: Retrospective cohort study.Neuropsychopharmacol Rep. 2024 Mar;44(1):60-66. doi: 10.1002/npr2.12378. Epub 2023 Sep 12. Neuropsychopharmacol Rep. 2024. PMID: 37698084 Free PMC article.
References
-
- NICE. Psychosis and Schizophrenia in Adults: The NICE Guideline on Treatment and Management. NICE Clinical Guideline 178; 2014. Available from: https://www.nice.org.uk/guidance/cg178. Accessed July 29, 2022.
LinkOut - more resources
Full Text Sources
Miscellaneous

