Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: the multinational observational SANTORINI study
- PMID: 37090089
- PMCID: PMC10119631
- DOI: 10.1016/j.lanepe.2023.100624
Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: the multinational observational SANTORINI study
Abstract
Background: European data pre-2019 suggest statin monotherapy is the most common approach to lipid management for preventing cardiovascular (CV) events, resulting in only one-fifth of high- and very high-risk patients achieving the 2019 ESC/EAS recommended low-density lipoprotein cholesterol (LDL-C) goals. Whether the treatment landscape has evolved, or gaps persist remains of interest.
Methods: Baseline data are presented from SANTORINI, an observational, prospective study that documents the use of lipid-lowering therapies (LLTs) in patients ≥18 years at high or very high CV risk between 2020 and 2021 across primary and secondary care settings in 14 European countries.
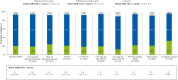
Findings: Of 9602 enrolled patients, 9044 with complete data were included (mean age: 65.3 ± 10.9 years; 72.6% male). Physicians reported using 2019 ESC/EAS guidelines as a basis for CV risk classification in 52.0% (4706/9044) of patients (overall: high risk 29.2%; very high risk 70.8%). However, centrally re-assessed CV risk based on 2019 ESC/EAS guidelines suggested 6.5% (308/4706) and 91.0% (4284/4706) were high- and very high-risk patients, respectively. Overall, 21.8% of patients had no documented LLTs, 54.2% were receiving monotherapy and 24.0% combination LLT. Median (interquartile range [IQR]) LDL-C was 2.1 (1.6, 3.0) mmol/L (82 [60, 117] mg/dL), with 20.1% of patients achieving risk-based LDL-C goals as per the 2019 ESC/EAS guidelines.
Interpretation: At the time of study enrolment, 80% of high- and very high-risk patients failed to achieve 2019 ESC/EAS guidelines LDL-C goals. Contributory factors may include CV risk underestimation and underutilization of combination therapies. Further efforts are needed to achieve current guideline-recommended LDL-C goals.
Trial registration: ClinicalTrials.gov Identifier: NCT04271280.
Funding: This study is funded by Daiichi Sankyo Europe GmbH, Munich, Germany.
Keywords: Cardiovascular disease; Cohort study; High cardiovascular risk; LDL cholesterol; Lipid-lowering therapy; Real-world evidence.
© 2023 The Author(s).
Conflict of interest statement
K.K.R. has received honoraria for consulting, lectures from Abbott Laboratories, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Cargene, CRISPR, Daiichi Sankyo, Eli Lilly Company, Esperion, Kowa, New Amsterdam Pharma, Novartis Corporation, Novo Nordisk, Pfizer, Regeneron, Sanofi, SCRIBE, Silence Therapeutics, and VAXXINITY. In addition, he has received research grant support to his institution from Amgen, Daiichi Sankyo, Sanofi and Regeneron. A.L.C. has received honoraria, lecture fees, or research grants from: Aegerion, Amgen, Amryt, AstraZeneca, Amarin, Daiichi Sankyo, Esperion, Ionis Pharmaceutical, Kowa, Medscape, Menarini, Merck, Mylan, Novartis, PeerVoice, Pfizer, Recordati, Regeneron, Sandoz, Sanofi, The Corpus. In addition, he has received research grants from Sanofi, Eli Lilly, Mylan, Sanofi Regeneron, Menarini, and Amgen. J.F. has received lecture fees from Akcea, Amgen, Lilly, Mylan, MSD, Sanofi and Servier. C.A. has received honoraria for consultancy and/or lectures fees from Abbott, Daiichi Sankyo, Novartis, Medinfar, and Tecnimede. In addition, he has received support from Tecnimede for attending meetings and/or travel. D.L.C. has received fees for advisory boards, research and lectures from, Daiichi Sankyo, and Novartis. T.S. has received consulting fees and research and educational grants from Amgen, Novartis, Orion Pharma, Pfizer, Sankyo, Sanofi and Servier. He has received payment or honoraria for lectures by profit and non-profit entities; support by the European Geriatric Medicine Society for attending meetings; he chaired the committee behind the publication of the National Finnish guideline for dyslipidaemia; lastly, he declared to be a statin patient. H.T. has received consulting and lecture fees as well as grants from Daiichi Sankyo. M.A. has received consulting and lecture fees from Alfasigma, Amgen, Amryt, IONIS/Akcea Therapeutics, Daiichi Sankyo, Novartis, Regeneron and Sanofi. He has also received research grants from Amgen, Amryt, IONIS/Akcea Therapeutics, Daiichi Sankyo, Novartis, Pfizer, Regeneron and Sanofi and payments for advisory board participation from Alfasigma, Amgen, Amryt, Pfizer. E.R. has received honoraria for lectures from Daiichi Sankyo, Servier and Novartis; payments for attending meetings from Sanofi; and for attending advisory boards from Amarin and Novartis; all paid directly to Ghent University. He is also President of the Belgian Atherosclerosis Society and a member of the Superior Health Council of Belgium, all voluntary roles. D.N. is or has been an investigator in clinical studies sponsored by Amgen, Pfizer, Daiichi Sankyo and Novartis; he has not received any personal fees for this work. J.M.M. has received lecture fees from Amgen, Daiichi Sankyo, Ferrer, Novartis, Servier, Sanofi and Viatris; and consultancy fees from Amgen, Sanofi, Novartis, Daiichi Sankyo, Servier. He has also received support from Sanofi and Amgen for attending meetings. U.L. has received honoraria for lectures and participation in advisory boards from Amgen, Daiichi Sankyo, Novartis and Sanofi. He has also received research grants to Leipzig University from Daiichi Sankyo, Amgen and Novartis, and is a member of DACH, DGK and ESC. I.H., M.C.M. and Aikaterini Bilitou are employees of Daiichi Sankyo and Annie Burden is a contract employee of Daiichi Sankyo. F.L.J.V. and M.E. declare no conflicts of interest.
Figures


References
-
- Townsend N., Wilson L., Bhatnagar P., Wickramasinghe K., Rayner M., Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–3245. - PubMed
-
- Ference B.A., Ginsberg H.N., Graham I., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. - PMC - PubMed
-
- Borén J., Chapman M.J., Krauss R.M., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313–2330. - PMC - PubMed
-
- Mach F., Baigent C., Catapano A.L., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur Heart J. 2020;41(1):111–188. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

