Caffeic acid, but not ferulic acid, inhibits macrophage pyroptosis by directly blocking gasdermin D activation
- PMID: 37090118
- PMCID: PMC10119582
- DOI: 10.1002/mco2.255
Caffeic acid, but not ferulic acid, inhibits macrophage pyroptosis by directly blocking gasdermin D activation
Abstract
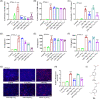
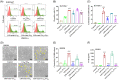
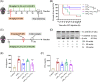
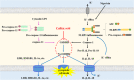
Regulated pyroptosis is critical for pathogen elimination by inducing infected cell rupture and pro-inflammatory cytokines secretion, while overwhelmed pyroptosis contributes to organ dysfunction and pathological inflammatory response. Caffeic acid (CA) and ferulic acid (FA) are both well-known antioxidant and anti-inflammatory phenolic acids, which resemble in chemical structure. Here we found that CA, but not FA, protects macrophages from both Nigericin-induced canonical and cytosolic lipopolysaccharide (LPS)-induced non-canonical pyroptosis and alleviates LPS-induced mice sepsis. It significantly improved the survival of pyroptotic cells and LPS-challenged mice and blocked proinflammatory cytokine secretion. The anti-pyroptotic effect of CA is independent of its regulations in cellular lipid peroxidation, mitochondrial function, or pyroptosis-associated gene transcription. Instead, CA arrests pyroptosis by directly associating with gasdermin D (GSDMD) and blocking its processing, resulting in reduced N-GSDMD pore construction and less cellular content release. In LPS-induced septic mice, CA inhibits GSDMD activation in peritoneal macrophages and reduces the serum levels of interleukin-1β and tumor necrosis factor-α as the known pyroptosis inhibitors, disulfiram and dimethyl fumarate. Collectively, these findings suggest that CA inhibits pyroptosis by targeting GSDMD and is a potential candidate for curbing the pyroptosis-associated disease.
Keywords: caffeic acid; ferulic acid; gasdermin D; macrophage; pyroptosis; sepsis.
© 2023 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
