Heterogeneity of adipose tissue-resident macrophages-beyond M1/M2 paradigm
- PMID: 37090127
- PMCID: PMC10113418
- DOI: 10.1007/s13340-023-00624-2
Heterogeneity of adipose tissue-resident macrophages-beyond M1/M2 paradigm
Abstract
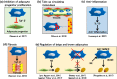
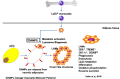
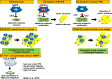
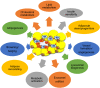
Adipose tissue-resident macrophages (ATMs) are reported to be important for maintaining adipose tissue remodeling and homeostasis. ATMs were classified for the first time in 2007 into the M1 and M2 types. This theory suggests that in the non-obese adipose tissue, the anti-inflammatory, alternatively activated macrophages (AAMs) predominate, and regulate tissue homeostasis, remodeling, and insulin sensitivity. On the other hand, classically activated M1-type macrophages increase rapidly in obesity, secrete inflammatory cytokines, such as TNFα and IL-6, and induce insulin resistance. In recent years, experimental findings that cannot be explained by this theory have been clarified one after another and the theory is being reconsidered. In this review, based on recent findings, we summarize reports on the novel metabolic regulatory functions of ATMs beyond the M1/M2 paradigm.
Keywords: Adipose tissue; Insulin resistance; M2 macrophage; Preadipocytes.
© The Japan Diabetes Society 2023, Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestK.T. received honoraria for lectures from MSD K.K. and scholarship grants from Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Company., Daiichi Sankyo Company, Ltd., Mitsubishi Tanabe Pharma Corporation, Asahi Kasei Pharma Corporation, Taisho Pharmaceutical Co., Ltd., Japan Diabetes Foundation and Japan Association for Diabetes Education and Care.
Figures