The Role of Cytosolic Lipid Droplets in Hepatitis C Virus Replication, Assembly, and Release
- PMID: 37090186
- PMCID: PMC10121354
- DOI: 10.1155/2023/5156601
The Role of Cytosolic Lipid Droplets in Hepatitis C Virus Replication, Assembly, and Release
Abstract
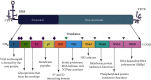
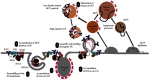
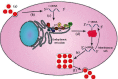
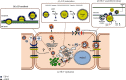
The hepatitis C virus (HCV) causes chronic hepatitis by establishing a persistent infection. Patients with chronic hepatitis frequently develop hepatic cirrhosis, which can lead to liver cancer-the progressive liver damage results from the host's immune response to the unresolved infection. The HCV replication process, including the entry, replication, assembly, and release stages, while the virus circulates in the bloodstream, it is intricately linked to the host's lipid metabolism, including the dynamic of the cytosolic lipid droplets (cLDs). This review article depicts how this interaction regulates viral cell tropism and aids immune evasion by coining viral particle characteristics. cLDs are intracellular organelles that store most of the cytoplasmic components of neutral lipids and are assumed to play an increasingly important role in the pathophysiology of lipid metabolism and host-virus interactions. cLDs are involved in the replication of several clinically significant viruses, where viruses alter the lipidomic profiles of host cells to improve viral life cycles. cLDs are involved in almost every phase of the HCV life cycle. Indeed, pharmacological modulators of cholesterol synthesis and intracellular trafficking, lipoprotein maturation, and lipid signaling molecules inhibit the assembly of HCV virions. Likewise, small-molecule inhibitors of cLD-regulating proteins inhibit HCV replication. Thus, addressing the molecular architecture of HCV replication will aid in elucidating its pathogenesis and devising preventive interventions that impede persistent infection and prevent disease progression. This is possible via repurposing the available therapeutic agents that alter cLDs metabolism. This review highlights the role of cLD in HCV replication.
Copyright © 2023 Abdullah A. Awadh.
Conflict of interest statement
The author declares that there is no conflict of interest regarding the publication of this article.
Figures

Similar articles
-
N-Myc Downstream-Regulated Gene 1 Restricts Hepatitis C Virus Propagation by Regulating Lipid Droplet Biogenesis and Viral Assembly.J Virol. 2018 Jan 2;92(2):e01166-17. doi: 10.1128/JVI.01166-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29118118 Free PMC article.
-
Complex lipid metabolic remodeling is required for efficient hepatitis C virus replication.Biochim Biophys Acta Mol Cell Biol Lipids. 2018 Sep;1863(9):1041-1056. doi: 10.1016/j.bbalip.2018.06.002. Epub 2018 Jun 6. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. PMID: 29885363
-
HCV Pit Stop at the Lipid Droplet: Refuel Lipids and Put on a Lipoprotein Coat before Exit.Cells. 2019 Mar 12;8(3):233. doi: 10.3390/cells8030233. Cells. 2019. PMID: 30871009 Free PMC article. Review.
-
Osteopontin Regulates Hepatitis C Virus (HCV) Replication and Assembly by Interacting with HCV Proteins and Lipid Droplets and by Binding to Receptors αVβ3 and CD44.J Virol. 2018 Jun 13;92(13):e02116-17. doi: 10.1128/JVI.02116-17. Print 2018 Jul 1. J Virol. 2018. PMID: 29669827 Free PMC article.
-
Hepatitis C Virus Replication.Adv Exp Med Biol. 2017;997:199-209. doi: 10.1007/978-981-10-4567-7_15. Adv Exp Med Biol. 2017. PMID: 28815532 Review.
Cited by
-
Lipid droplets in pathogen infection and host immunity.Acta Pharmacol Sin. 2024 Mar;45(3):449-464. doi: 10.1038/s41401-023-01189-1. Epub 2023 Nov 22. Acta Pharmacol Sin. 2024. PMID: 37993536 Free PMC article. Review.
-
Dyslipidemia in severe fever with thrombocytopenia syndrome patients: A retrospective cohort study.PLoS Negl Trop Dis. 2024 Dec 11;18(12):e0012673. doi: 10.1371/journal.pntd.0012673. eCollection 2024 Dec. PLoS Negl Trop Dis. 2024. PMID: 39661593 Free PMC article.
-
Mitochondrial Dysfunction and Metabolic Disturbances Induced by Viral Infections.Cells. 2024 Oct 29;13(21):1789. doi: 10.3390/cells13211789. Cells. 2024. PMID: 39513896 Free PMC article. Review.
-
Liposome-Mediated Anti-Viral Drug Delivery Across Blood-Brain Barrier: Can Lipid Droplet Target Be Game Changers?Cell Mol Neurobiol. 2023 Dec 20;44(1):9. doi: 10.1007/s10571-023-01443-4. Cell Mol Neurobiol. 2023. PMID: 38123863 Free PMC article. Review.
-
Metabolic reprogramming in viral infections: the interplay of glucose metabolism and immune responses.Front Immunol. 2025 May 16;16:1578202. doi: 10.3389/fimmu.2025.1578202. eCollection 2025. Front Immunol. 2025. PMID: 40453076 Free PMC article. Review.
References
-
- World Health Organization. Hepatitis C. 2022, http://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

