Spermidine as a promising anticancer agent: Recent advances and newer insights on its molecular mechanisms
- PMID: 37090250
- PMCID: PMC10117651
- DOI: 10.3389/fchem.2023.1164477
Spermidine as a promising anticancer agent: Recent advances and newer insights on its molecular mechanisms
Abstract
Spermidine is a naturally occurring polyamine compound found in semen. It is also found in several plant sources and boasts a remarkable biological profile, particularly with regards to its anticancer properties. Spermidine specifically interferes with the tumour cell cycle, resulting in the inhibition of tumor cell proliferation and suppression of tumor growth. Moreover, it also triggers autophagy by regulating key oncologic pathways. The increased intake of polyamines, such as spermidine, can suppress oncogenesis and slow the growth of tumors due to its role in anticancer immunosurveillance and regulation of polyamine metabolism. Spermidine/spermine N-1-acetyltransferase (SSAT) plays a critical role in polyamine homeostasis and serves as a diagnostic marker in human cancers. Chemically modified derivatives of spermidine hold great potential for prognostic, diagnostic, and therapeutic applications against various malignancies. This review discusses in detail the recent findings that support the anticancer mechanisms of spermidine and its molecular physiology.
Keywords: anticancer immunosurveillance; anticancer properties; cell proliferation; diagnostic marker; polyamines; spermidine.
Copyright © 2023 Prasher, Sharma, Singh, Gulati, Chellappan, Rajput, Gupta, Ydyrys, Kulbayeva, Abdull Razis, Modu, Sharifi-Rad and Dua.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




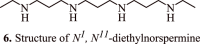



References
-
- Akyol Z., Coker-Gurkan A., Arisan E. D., Obakan-Yerlikaya P., Palavan-Unsal N. (2016). DENSpm overcame Bcl-2 mediated resistance against Paclitaxel treatment in MCF-7 breast cancer cells via activating polyamine catabolic machinery. Biomed. Pharmacother. 84, 2029–2041. 10.1016/j.biopha.2016.11.016 - DOI - PubMed
-
- Bernacki R. J., Bergeron R. J., Porter C. W. (1992). Antitumor activity of N,N'-bis(ethyl)spermine homologues against human MALME-3 melanoma xenografts. Cancer Res. 52, 2424–2430. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

