Circulating extracellular vesicles promote recovery in a preclinical model of intracerebral hemorrhage
- PMID: 37090418
- PMCID: PMC10113711
- DOI: 10.1016/j.omtn.2023.03.006
Circulating extracellular vesicles promote recovery in a preclinical model of intracerebral hemorrhage
Abstract
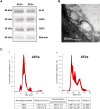
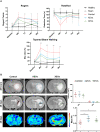
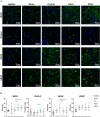
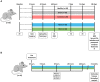
Circulating extracellular vesicles (EVs) are proposed to participate in enhancing pathways of recovery after stroke through paracrine signaling. To verify this hypothesis in a proof-of-concept study, blood-derived allogenic EVs from rats and xenogenic EVs from humans who experienced spontaneous good recovery after an intracerebral hemorrhage (ICH) were administered intravenously to rats at 24 h after a subcortical ICH. At 28 days, both treatments improved the motor function assessment scales score, showed greater fiber preservation in the perilesional zone (diffusion tensor-fractional anisotropy MRI), increased immunofluorescence markers of myelin (MOG), and decreased astrocyte markers (GFAP) compared with controls. Comparison of the protein cargo of circulating EVs at 28 days from animals with good vs. poor recovery showed down-expression of immune system activation pathways (CO4, KLKB1, PROC, FA9, and C1QA) and of restorative processes such as axon guidance (RAC1), myelination (MBP), and synaptic vesicle trafficking (SYN1), which is in line with better tissue preservation. Up-expression of PCSK9 (neuron differentiation) in xenogenic EVs-treated animals suggests enhancement of repair pathways. In conclusion, the administration of blood-derived EVs improved recovery after ICH. These findings open a new and promising opportunity for further development of restorative therapies to improve the outcomes after an ICH.
Keywords: MT: Special Issue - Exploiting Extracellular Vesicles as Therapeutic Agents; brain repair; extracellular vesicles; intracerebral hemorrhage; preclinical studies; proteomics; safety; treatment.
© 2023 The Author(s).
Conflict of interest statement
A.G.-P. is a shareholder of Biomedica Molecular Medicine SL.
Figures


References
-
- Krishnamurthi R.V., Feigin V.L., Forouzanfar M.H., Mensah G.A., Connor M., Bennett D.A., Moran A.E., Sacco R.L., Anderson L.M., Truelsen T., et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. Glob. Health. 2013;1:e259–e281. doi: 10.1016/S2214-109X(13)70089-5. - DOI - PMC - PubMed
-
- GBD 2016 Stroke Collaborators. Nguyen M., Roth G.A., Nichols E., Alam T., Abate D., Abd-Allah F., Abdelalim A., Abraha H.N., Abu-Rmeileh N.M., et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

