This is a preprint.
Optogenetic-Induced Muscle Loading Leads to Mechanical Adaptation of the Achilles Tendon Enthesis in Mice
- PMID: 37090593
- PMCID: PMC10120626
- DOI: 10.1101/2023.04.11.536376
Optogenetic-Induced Muscle Loading Leads to Mechanical Adaptation of the Achilles Tendon Enthesis in Mice
Update in
-
Optogenetic-induced muscle loading leads to mechanical adaptation of the Achilles tendon enthesis in mice.Sci Adv. 2023 Jun 23;9(25):eadf4683. doi: 10.1126/sciadv.adf4683. Epub 2023 Jun 23. Sci Adv. 2023. PMID: 37352350 Free PMC article.
Abstract
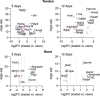
The growth of the skeleton depends on the transmission of contractile muscle forces from tendon to bone across the extracellular matrix-rich enthesis. Loss of muscle loading leads to significant impairments in enthesis development. However, little is known about how the enthesis responds to increased loading during postnatal growth. To study the cellular and matrix adaptations of the enthesis in response to increased muscle loading, we used optogenetics to induce skeletal muscle contraction and unilaterally load the Achilles tendon and enthesis in young (i.e., during growth) and adult (i.e., mature) mice. In young mice, daily bouts of unilateral optogenetic loading led to expansion of the calcaneal apophysis and growth plate, as well as increased vascularization of the normally avascular enthesis. Daily loading bouts, delivered for 3 weeks, also led to a mechanically weaker enthesis with increased molecular-level accumulation of collagen damage in young mice. However, adult mice did not exhibit impaired mechanical properties or noticeable structural adaptations to the enthesis. We then focused on the transcriptional response of the young tendon and bone following optogenetic-induced loading. After 1 or 2 weeks of loading, we identified, in tendon, transcriptional activation of canonical pathways related to glucose metabolism (glycolysis) and inhibited pathways associated with cytoskeletal remodeling (e.g., RHOA and CREB signaling). In bone, we identified activation of inflammatory signaling (e.g., NFkB and STAT3 signaling) and inhibition of ERK/MAPK and PTEN signaling. Thus, we have demonstrated the utility of optogenetic-induced skeletal muscle contraction to elicit structural, functional, and molecular adaptation of the enthesis in vivo especially during growth.
Figures




References
-
- Achar Suraj, and Yamanaka Jarrod. 2019. “Apophysitis and Osteochondrosis: Common Causes of Pain in Growing Bones.” American Family Physician 99 (10): 610–18. - PubMed
-
- Andarawis-Puri Nelly, Sereysky Jedd B., Jepsen Karl J., and Flatow Evan L.. 2012. “The Relationships between Cyclic Fatigue Loading, Changes in Initial Mechanical Properties, and the in Vivo Temporal Mechanical Response of the Rat Patellar Tendon.” Journal of Biomechanics 45 (1): 59–65. 10.1016/j.jbiomech.2011.10.008. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
