Fibronectin leucine-rich transmembrane protein 2 drives monocyte differentiation into macrophages via the UNC5B-Akt/mTOR axis
- PMID: 37090697
- PMCID: PMC10117657
- DOI: 10.3389/fimmu.2023.1162004
Fibronectin leucine-rich transmembrane protein 2 drives monocyte differentiation into macrophages via the UNC5B-Akt/mTOR axis
Abstract
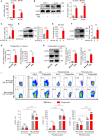
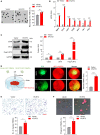
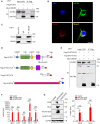
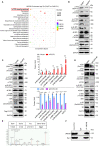
Upon migrating into the tissues, hematopoietic stem cell (HSC)-derived monocytes differentiate into macrophages, playing a crucial role in determining innate immune responses towards external pathogens and internal stimuli. However, the regulatory mechanisms underlying monocyte-to-macrophage differentiation remain largely unexplored. Here we divulge a previously uncharacterized but essential role for an axon guidance molecule, fibronectin leucine-rich transmembrane protein 2 (FLRT2), in monocyte-to-macrophage maturation. FLRT2 is almost undetectable in human monocytic cell lines, human peripheral blood mononuclear cells (PBMCs), and mouse primary monocytes but significantly increases in fully differentiated macrophages. Myeloid-specific deletion of FLRT2 (Flrt2ΔMyel ) contributes to decreased peritoneal monocyte-to-macrophage generation in mice in vivo, accompanied by impaired macrophage functions. Gain- and loss-of-function studies support the promoting effect of FLRT2 on THP-1 cell and human PBMC differentiation into macrophages. Mechanistically, FLRT2 directly interacts with Unc-5 netrin receptor B (UNC5B) via its extracellular domain (ECD) and activates Akt/mTOR signaling. In vivo administration of mTOR agonist MYH1485 reverses the impaired phenotypes observed in Flrt2ΔMyel mice. Together, these results identify FLRT2 as a novel pivotal endogenous regulator of monocyte differentiation into macrophages. Targeting the FLRT2/UNC5B-Akt/mTOR axis may provide potential therapeutic strategies directly relevant to human diseases associated with aberrant monocyte/macrophage differentiation.
Keywords: FLRT2; MTOR signaling; UNC5B; differentiation; macrophage; monocyte.
Copyright © 2023 Fang, Ma, Huang, Dang, Liu, Xu, Zheng, Yang, Huo and Dai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JW declared a shared affiliation with the author, XY, to the handling editor at the time of the review.
Figures



Similar articles
-
Flrt2 is involved in fine-tuning of osteoclast multinucleation.BMB Rep. 2019 Aug;52(8):514-519. doi: 10.5483/BMBRep.2019.52.8.116. BMB Rep. 2019. PMID: 31383250 Free PMC article.
-
Targeting Unc5b in macrophages drives atherosclerosis regression and pro-resolving immune cell function.Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2412690121. doi: 10.1073/pnas.2412690121. Epub 2024 Oct 22. Proc Natl Acad Sci U S A. 2024. PMID: 39436659 Free PMC article.
-
FLRT2 and FLRT3 act as repulsive guidance cues for Unc5-positive neurons.EMBO J. 2011 Jun 14;30(14):2920-33. doi: 10.1038/emboj.2011.189. EMBO J. 2011. PMID: 21673655 Free PMC article.
-
[Recent studies on PI3K/AKT/mTOR signaling pathway in hematopoietic stem cells].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013 Feb;21(1):245-9. doi: 10.7534/j.issn.1009-2137.2013.01.050. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013. PMID: 23484729 Review. Chinese.
-
The Complexity of Targeting PI3K-Akt-mTOR Signalling in Human Acute Myeloid Leukaemia: The Importance of Leukemic Cell Heterogeneity, Neighbouring Mesenchymal Stem Cells and Immunocompetent Cells.Molecules. 2016 Nov 11;21(11):1512. doi: 10.3390/molecules21111512. Molecules. 2016. PMID: 27845732 Free PMC article. Review.
Cited by
-
Roles of PANoptosis and related genes in acute liver failure: neoteric insight from bioinformatics analysis and animal experiment verification.J Zhejiang Univ Sci B. 2025 Apr 23;26(4):353-370. doi: 10.1631/jzus.B2300678. J Zhejiang Univ Sci B. 2025. PMID: 40274384 Free PMC article.
-
Single-cell transcriptomic profiling reveals a novel signature of necrotizing granulomatous lesions in the lungs of Mycobacterium tuberculosis-infected C3HeB/FeJ mice.Front Immunol. 2025 Aug 6;16:1624072. doi: 10.3389/fimmu.2025.1624072. eCollection 2025. Front Immunol. 2025. PMID: 40843005 Free PMC article.
-
CD4+ T Cell Subsets and PTPN22 as Novel Biomarkers of Immune Dysregulation in Dilated Cardiomyopathy.Int J Mol Sci. 2025 Aug 13;26(16):7806. doi: 10.3390/ijms26167806. Int J Mol Sci. 2025. PMID: 40869128 Free PMC article.
-
A protocol to isolate and characterize pure monocytes and generate monocyte-derived dendritic cells through FBS-Coated flasks.Sci Rep. 2024 Oct 14;14(1):23956. doi: 10.1038/s41598-024-75376-3. Sci Rep. 2024. PMID: 39397067 Free PMC article.
-
Interactions between Schwann cell and extracellular matrix in peripheral nerve regeneration.Front Neurol. 2024 Mar 26;15:1372168. doi: 10.3389/fneur.2024.1372168. eCollection 2024. Front Neurol. 2024. PMID: 38651098 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

