Syphilis vaccine: challenges, controversies and opportunities
- PMID: 37090699
- PMCID: PMC10118025
- DOI: 10.3389/fimmu.2023.1126170
Syphilis vaccine: challenges, controversies and opportunities
Abstract
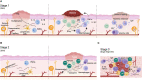
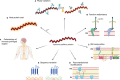
Syphilis is a sexually or vertically (mother to fetus) transmitted disease caused by the infection of Treponema pallidum subspecie pallidum (TPA). The incidence of syphilis has increased over the past years despite the fact that this bacterium is an obligate human pathogen, the infection route is well known, and the disease can be successfully treated with penicillin. As complementary measures to preventive campaigns and early treatment of infected individuals, development of a syphilis vaccine may be crucial for controlling disease spread and/or severity, particularly in countries where the effectiveness of the aforementioned measures is limited. In the last century, several vaccine prototypes have been tested in preclinical studies, mainly in rabbits. While none of them provided protection against infection, some prototypes prevented bacteria from disseminating to distal organs, attenuated lesion development, and accelerated their healing. In spite of these promising results, there is still some controversy regarding the identification of vaccine candidates and the characteristics of a syphilis-protective immune response. In this review, we describe what is known about TPA immune response, and the main mechanisms used by this pathogen to evade it. Moreover, we emphasize the importance of integrating this knowledge, in conjunction with the characterization of outer membrane proteins (OMPs), to expedite the development of a syphilis vaccine that can protect against TPA infection.
Keywords: immune response; outer membrane proteins; syphilis; treponema pallidum; vaccine.
Copyright © 2023 Ávila-Nieto, Pedreño-López, Mitjà, Clotet, Blanco and Carrillo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

