Severe COVID-19 and non-COVID-19 severe sepsis converge transcriptionally after a week in the intensive care unit, indicating common disease mechanisms
- PMID: 37090709
- PMCID: PMC10115984
- DOI: 10.3389/fimmu.2023.1167917
Severe COVID-19 and non-COVID-19 severe sepsis converge transcriptionally after a week in the intensive care unit, indicating common disease mechanisms
Abstract
Introduction: Severe COVID-19 and non-COVID-19 pulmonary sepsis share pathophysiological, immunological, and clinical features. To what extent they share mechanistically-based gene expression trajectories throughout hospitalization was unknown. Our objective was to compare gene expression trajectories between severe COVID-19 patients and contemporaneous non-COVID-19 severe sepsis patients in the intensive care unit (ICU).
Methods: In this prospective single-center observational cohort study, whole blood was drawn from 20 COVID-19 patients and 22 non-COVID-19 adult sepsis patients at two timepoints: ICU admission and approximately a week later. RNA-Seq was performed on whole blood to identify differentially expressed genes and significantly enriched pathways.
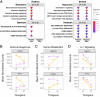
Results: At ICU admission, despite COVID-19 patients being almost clinically indistinguishable from non-COVID-19 sepsis patients, COVID-19 patients had 1,215 differentially expressed genes compared to non-COVID-19 sepsis patients. After one week in the ICU, the number of differentially expressed genes dropped to just 9 genes. This drop coincided with decreased expression of antiviral genes and relatively increased expression of heme metabolism genes over time in COVID-19 patients, eventually reaching expression levels seen in non-COVID-19 sepsis patients. Both groups also had similar underlying immune dysfunction, with upregulation of immune processes such as "Interleukin-1 signaling" and "Interleukin-6/JAK/STAT3 signaling" throughout disease compared to healthy controls.
Discussion: Early on, COVID-19 patients had elevated antiviral responses and suppressed heme metabolism processes compared to non-COVID-19 severe sepsis patients, although both had similar underlying immune dysfunction. However, after one week in the ICU, these diseases became indistinguishable on a gene expression level. These findings highlight the importance of early antiviral treatment for COVID-19, the potential for heme-related therapeutics, and consideration of immunomodulatory therapies for both diseases to treat shared immune dysfunction.
Keywords: COVID-19; gene expression; immune dysfunction; longitudinal analyses; sepsis.
Copyright © 2023 An, Baghela, Zhang, Falsafi, Lee, Trahtemberg, Baker, dos Santos and Hancock.
Conflict of interest statement
RH has a significant ownership position in Sepset Biotherapeutics Inc and has filed patents for sepsis diagnostic assays an indirect relationship to this work. CS is on the Data and Safety Monitoring Board of SEMPATICO NCT04615871. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Coronavirus statistics. In: Worldometer. Available at: https://www.worldometers.info/coronavirus/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

