The small molecule inhibitor BX-795 uncouples IL-2 production from inhibition of Th2 inflammation and induces CD4+ T cells resembling iTreg
- PMID: 37090735
- PMCID: PMC10117943
- DOI: 10.3389/fimmu.2023.1094694
The small molecule inhibitor BX-795 uncouples IL-2 production from inhibition of Th2 inflammation and induces CD4+ T cells resembling iTreg
Abstract
Background: Treg cells have been shown to be an important part of immune-homeostasis and IL-2 which is produced upon T cell receptor (TCR)-dependent activation of T lymphocytes has been demonstrated to critically participate in Treg development.
Objective: To evaluate small molecule inhibitors (SMI) for the identification of novel IL-2/Treg enhancing compounds.
Materials and methods: We used TCR-dependent and allergen-specific cytokine secretion of human and mouse T cells, next generation messenger ribonucleic acid sequencing (RNA-Seq) and two different models of allergic airway inflammation to examine lead SMI-compounds.
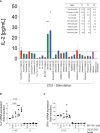
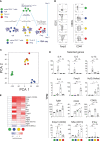
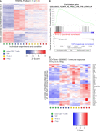
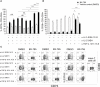
Results: We show here that the reported 3-phosphoinositide dependent kinase-1 (PDK1) SMI BX-795 increased IL-2 in culture supernatants of Jurkat E6-1 T cells, human peripheral blood mononuclear cells (hPBMC) and allergen-specific mouse T cells upon TCR-dependent and allergen-specific stimulation while concomitantly inhibiting Th2 cytokine secretion. RNA-Seq revealed that the presence of BX-795 during allergen-specific activation of T cells induces a bona fide Treg cell type highly similar to iTreg but lacking Foxp3 expression. When applied in mugwort pollen and house dust mite extract-based models of airway inflammation, BX-795 significantly inhibited Th2 inflammation including expression of Th2 signature transcription factors and cytokines and influx into the lungs of type 2-associated inflammatory cells such as eosinophils.
Conclusions: BX-795 potently uncouples IL-2 production from Th2 inflammation and induces Th-IL-2 cells, which highly resemble induced (i)Tregs. Thus, BX-795 may be a useful new compound for the treatment of allergic diseases.
Keywords: IL-2; Immunomodulation; Th2 cells; allergy; regulatory T cells; small molecule inhibitor.
Copyright © 2023 Tauber, Kratzer, Schatzlmaier, Smole, Köhler, Rausch, Kranich, Trapin, Neunkirchner, Zabel, Jutz, Steinberger, Gadermaier, Brocker, Stockinger, Derdak and Pickl.
Conflict of interest statement
With regards to the authors disclosure of potential conflicts of interest we would like to indicate that WP received honoraria from Novartis, Astra Zeneca and Roche, GSK and Bristol-Myers Squibb outside the submitted work and GG reports receiving personal fees from Bencard, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta.PLoS Pathog. 2009 Aug;5(8):e1000543. doi: 10.1371/journal.ppat.1000543. Epub 2009 Aug 14. PLoS Pathog. 2009. PMID: 19680449 Free PMC article.
-
Protein kinase Cθ controls type 2 innate lymphoid cell and TH2 responses to house dust mite allergen.J Allergy Clin Immunol. 2017 May;139(5):1650-1666. doi: 10.1016/j.jaci.2016.08.044. Epub 2016 Oct 14. J Allergy Clin Immunol. 2017. PMID: 27746240
-
Phenotype analyses of IL-10-producing Foxp3- CD4+ T cells increased by subcutaneous immunotherapy in allergic airway inflammation.Int Immunopharmacol. 2018 Aug;61:297-305. doi: 10.1016/j.intimp.2018.06.014. Epub 2018 Jun 14. Int Immunopharmacol. 2018. PMID: 29909233
-
Novel cytokines and cytokine-producing T cells in allergic disorders.Allergy Asthma Proc. 2011 Mar-Apr;32(2):83-94. doi: 10.2500/aap.2011.32.3428. Allergy Asthma Proc. 2011. PMID: 21439160 Review.
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
Cited by
-
The T-Cell Growth Factor Interleukin-2, Which Is Occasionally Targeted by Autoantibodies, Qualifies as Drug for the Treatment of Allergy, Autoimmunity, and Cancer: Collegium Internationale Allergologicum (CIA) Update 2024.Int Arch Allergy Immunol. 2024;185(3):286-300. doi: 10.1159/000533677. Epub 2023 Dec 12. Int Arch Allergy Immunol. 2024. PMID: 38086339 Free PMC article. Review.
-
Small Molecule Targeting Immune Cells: A Novel Approach for Cancer Treatment.Biomedicines. 2023 Sep 24;11(10):2621. doi: 10.3390/biomedicines11102621. Biomedicines. 2023. PMID: 37892995 Free PMC article. Review.
References
-
- Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. . IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood (2006) 108:1571–9. doi: 10.1182/blood-2006-02-004747 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

