Mode of presentation and performance of serology assays for diagnosing celiac disease: A single-center study in the United Arab Emirates
- PMID: 37090770
- PMCID: PMC10113562
- DOI: 10.3389/fnut.2023.1107017
Mode of presentation and performance of serology assays for diagnosing celiac disease: A single-center study in the United Arab Emirates
Abstract
Objective: To characterize patients with celiac disease (CD), examines the clinical spectrum of CD, and evaluate the performance of serologic tests used for CD screening, in the United Arab Emirates (UAE).
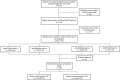
Methods: Medical charts of patients received at the Digestive Diseases Institute of Cleveland Clinic Abu Dhabi from January 2015 to December 2020 were reviewed. Patients who were screened for four serologic biomarkers (anti-tissue transglutaminase IgA [Anti-tTG-IgA], anti-tissue transglutaminase IgG [Anti-TtG-IgG], anti-deamidated gliadin peptide IgG [Anti-DGP-IgG], and anti-deamidated gliadin peptide IgA [Anti-DGP-IgA]) were included. Histopathology was performed on patients with the seropositive test. Marsh score > 1 considered to confirm CD. Characteristics of the Anti-tTG-IgA seropositive patients were described and that correlated with histopathologically confirmed CD were explored.
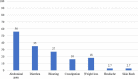
Results: Of the 6,239 patients, 1.4, 2.9, 4.7, and 4.9%, were seropositive to Anti-tTG-IgG, Anti-TtG-IgA, Anti-DGP-IgA, and Anti-DGP-IgG, respectively. Overall, 7.7% were seropositive to either of the four biomarkers. Of the biopsy-screened 300 patients, 38.7% (1.9% of the total serologically screened) were confirmed with CD. The mean age of Anti-TtG-IgA seropositive patients was 32.1 ± 10.3 SD years, 72% of them were females, and 93.4% were Emirati. In those patients, overweight (28.7%) and obesity (24.7%) were common while 5.8% of patients were underweight. Anemia prevalence was 46.7%, 21.3% had Gastroesophageal reflux disease (GERD), 7.7% with autoimmune thyroid disease, 5.5% (type 1), and 3.3% (type 2) were diabetic. Vitamin D deficiency was observed in 47.8% of the Anti-TtG IgA seropositive patients. Twelve (10.3%) histopathologically confirmed CD patients were seronegative to Anti-TtG-IgA but seropositive to anti-DGP-IgA and/or Anti-DGP-IgG. Body mass index, GERD, autoimmune thyroid disease, type 1 diabetes, asthma, hemoglobin, and vitamin D concentration, were all correlated with biopsy-confirmed CD (P < 0.05). Compared to the gold-standard biopsy test, Anti-TtG-IgA had the highest sensitivity (89.7%) and specificity (83.7%).
Conclusion: Three and two of every 100 patients were serologically (anti-tTG-IgA positive) and histopathologically diagnosed with CD, respectively. Although Anti-TtG-IgA is the most sensitive, specific, and commonly used test, one of every ten histopathologically confirmed patients and Anti-tTG-IgA seronegative were seropositive to Anti-DGP. To avoid missing patients with CD, a comprehensive serological investigation covering DGP-IgG/IgA is warranted.
Keywords: Cleveland Clinic; United Arab Emirates; celiac disease; duodenal biopsy; gastroenterology; serologic biomarkers.
Copyright © 2023 Shatnawei, AlNababteh, Govender, Al-Shamsi, AlJarrah and Al-Rifai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Performance of deamidated gliadin peptide antibodies as first screening for celiac disease in the general pediatric population.Front Pediatr. 2023 Nov 21;11:1279825. doi: 10.3389/fped.2023.1279825. eCollection 2023. Front Pediatr. 2023. PMID: 38078323 Free PMC article.
-
No-Biopsy Diagnosis of Coeliac Disease in Children Without Anti-Endomysial IgA Antibody Testing: Combining Anti-Tissue Transglutaminase IgA and Anti-Deamidated Gliadin IgG Antibodies.J Paediatr Child Health. 2025 Apr;61(4):628-634. doi: 10.1111/jpc.16801. Epub 2025 Jan 31. J Paediatr Child Health. 2025. PMID: 39888493 Free PMC article.
-
Diagnostic Value of Immunoglobulin G Anti-Deamidated Gliadin Peptide Antibody for Diagnosis of Pediatric Celiac Disease: A Study from Shiraz, Iran.Pediatr Gastroenterol Hepatol Nutr. 2022 Jul;25(4):312-320. doi: 10.5223/pghn.2022.25.4.312. Epub 2022 Jul 6. Pediatr Gastroenterol Hepatol Nutr. 2022. PMID: 35903491 Free PMC article.
-
The role of serology in the diagnosis of coeliac disease.Gastroenterol Hepatol Bed Bench. 2023;16(2):118-128. doi: 10.22037/ghfbb.v16i2.2713. Gastroenterol Hepatol Bed Bench. 2023. PMID: 37554756 Free PMC article. Review.
-
Diagnostic Accuracy of IgA Anti-Transglutaminase and IgG Anti-Deamidated Gliadin for Diagnosis of Celiac Disease in Children under Two Years of Age: A Systematic Review and Meta-Analysis.Nutrients. 2021 Dec 21;14(1):7. doi: 10.3390/nu14010007. Nutrients. 2021. PMID: 35010880 Free PMC article.
References
-
- Chou R, Blazina I, Bougatsos C, Mackey K, Grusing S, Selph S. U.S. Preventive Services Task Force Evidence Syntheses, Formerly Systematic Evidence Reviews. Screening for Celiac Disease: A Systematic Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US) (2017). 10.1001/jama.2016.10395 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous