Opioids, sleep, analgesia and respiratory depression: Their convergence on Mu (μ)-opioid receptors in the parabrachial area
- PMID: 37090798
- PMCID: PMC10117663
- DOI: 10.3389/fnins.2023.1134842
Opioids, sleep, analgesia and respiratory depression: Their convergence on Mu (μ)-opioid receptors in the parabrachial area
Abstract
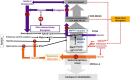
Opioids provide analgesia, as well as modulate sleep and respiration, all by possibly acting on the μ-opioid receptors (MOR). MOR's are ubiquitously present throughout the brain, posing a challenge for understanding the precise anatomical substrates that mediate opioid induced respiratory depression (OIRD) that ultimately kills most users. Sleep is a major modulator not only of pain perception, but also for changing the efficacy of opioids as analgesics. Therefore, sleep disturbances are major risk factors for developing opioid overuse, withdrawal, poor treatment response for pain, and addiction relapse. Despite challenges to resolve the neural substrates of respiratory malfunctions during opioid overdose, two main areas, the pre-Bötzinger complex (preBötC) in the medulla and the parabrachial (PB) complex have been implicated in regulating respiratory depression. More recent studies suggest that it is mediation by the PB that causes OIRD. The PB also act as a major node in the upper brain stem that not only receives input from the chemosensory areas in medulla, but also receives nociceptive information from spinal cord. We have previously shown that the PB neurons play an important role in mediating arousal from sleep in response to hypercapnia by its projections to the forebrain arousal centers, and it may also act as a major relay for the pain stimuli. However, due to heterogeneity of cells in the PB, their precise roles in regulating, sleep, analgesia, and respiratory depression, needs addressing. This review sheds light on interactions between sleep and pain, along with dissecting the elements that adversely affects respiration.
Keywords: analgesia; opioid induced respiratory depression; opioid tolerance; opioid use disorder; sleep-loss.
Copyright © 2023 Lynch, Lima, Spinieli and Kaur.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Current research in pathophysiology of opioid-induced respiratory depression, neonatal opioid withdrawal syndrome, and neonatal antidepressant exposure syndrome.Curr Res Toxicol. 2022 Jun 6;3:100078. doi: 10.1016/j.crtox.2022.100078. eCollection 2022. Curr Res Toxicol. 2022. PMID: 35734228 Free PMC article.
-
Mechanisms of opioid-induced respiratory depression.Arch Toxicol. 2022 Aug;96(8):2247-2260. doi: 10.1007/s00204-022-03300-7. Epub 2022 Apr 26. Arch Toxicol. 2022. PMID: 35471232 Review.
-
Opioid suppression of an excitatory pontomedullary respiratory circuit by convergent mechanisms.Elife. 2023 Jun 14;12:e81119. doi: 10.7554/eLife.81119. Elife. 2023. PMID: 37314062 Free PMC article.
-
PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression.J Neurosci. 2011 Jan 26;31(4):1292-301. doi: 10.1523/JNEUROSCI.4611-10.2011. J Neurosci. 2011. PMID: 21273414 Free PMC article.
-
Opioid-induced respiratory depression: clinical aspects and pathophysiology of the respiratory network effects.Am J Physiol Lung Cell Mol Physiol. 2025 Feb 1;328(2):L267-L289. doi: 10.1152/ajplung.00314.2024. Epub 2024 Dec 27. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 39726397 Review.
Cited by
-
Identifying the Brain Circuits that Regulate Pain-Induced Sleep Disturbances.bioRxiv [Preprint]. 2024 Dec 20:2024.12.20.629596. doi: 10.1101/2024.12.20.629596. bioRxiv. 2024. PMID: 39763835 Free PMC article. Preprint.
-
Modeling Effects of Variable preBötzinger Complex Network Topology and Cellular Properties on Opioid-Induced Respiratory Depression and Recovery.eNeuro. 2024 Mar 7;11(3):ENEURO.0284-23.2023. doi: 10.1523/ENEURO.0284-23.2023. Print 2024 Mar. eNeuro. 2024. PMID: 38253582 Free PMC article.
-
Sleep-Disordered Breathing and Interactions with Opioids: A Narrative Review.J Clin Med. 2025 Jul 4;14(13):4758. doi: 10.3390/jcm14134758. J Clin Med. 2025. PMID: 40649133 Free PMC article. Review.
-
Respiratory Depression Associated with Opioids: A Narrative Review.Curr Treat Options Oncol. 2024 Nov;25(11):1438-1450. doi: 10.1007/s11864-024-01274-5. Epub 2024 Oct 21. Curr Treat Options Oncol. 2024. PMID: 39432171 Review.
-
The Rise of Fentanyl: Molecular Aspects and Forensic Investigations.Int J Mol Sci. 2025 Jan 7;26(2):444. doi: 10.3390/ijms26020444. Int J Mol Sci. 2025. PMID: 39859160 Free PMC article. Review.
References
-
- Alexandre C., Latremolier A., Finan P. (2020). “Effect of sleep loss on pain,” in The Oxford Handbook of the Neurobiology of Pain, ed. Wood J. N. (Oxford: Oxford University Press; ), 556–608.
-
- Ardon A., Gillespie N., Kolli S., Shilling A. M., Warrick M. (2020). Patient-controlled analgesia in high-risk populations: Implications for safety. Curr. Anesthesiol. Rep. 10 463–472.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

