Neuroprotective effects of resistance physical exercise on the APP/PS1 mouse model of Alzheimer's disease
- PMID: 37090809
- PMCID: PMC10116002
- DOI: 10.3389/fnins.2023.1132825
Neuroprotective effects of resistance physical exercise on the APP/PS1 mouse model of Alzheimer's disease
Abstract
Introduction: Physical exercise has beneficial effects by providing neuroprotective and anti-inflammatory responses to AD. Most studies, however, have been conducted with aerobic exercises, and few have investigated the effects of other modalities that also show positive effects on AD, such as resistance exercise (RE). In addition to its benefits in developing muscle strength, balance and muscular endurance favoring improvements in the quality of life of the elderly, RE reduces amyloid load and local inflammation, promotes memory and cognitive improvements, and protects the cortex and hippocampus from the degeneration that occurs in AD. Similar to AD patients, double-transgenic APPswe/PS1dE9 (APP/PS1) mice exhibit Αβ plaques in the cortex and hippocampus, hyperlocomotion, memory deficits, and exacerbated inflammatory response. Therefore, the aim of this study was to investigate the effects of 4 weeks of RE intermittent training on the prevention and recovery from these AD-related neuropathological conditions in APP/PS1 mice.
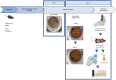
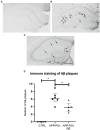
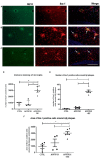
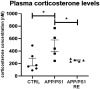
Methods: For this purpose, 6-7-month-old male APP/PS1 transgenic mice and their littermates, negative for the mutations (CTRL), were distributed into three groups: CTRL, APP/PS1, APP/PS1+RE. RE training lasted four weeks and, at the end of the program, the animals were tested in the open field test for locomotor activity and in the object recognition test for recognition memory evaluation. The brains were collected for immunohistochemical analysis of Aβ plaques and microglia, and blood was collected for plasma corticosterone by ELISA assay.
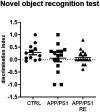
Results: APP/PS1 transgenic sedentary mice showed increased hippocampal Aβ plaques and higher plasma corticosterone levels, as well as hyperlocomotion and reduced central crossings in the open field test, compared to APP/PS1 exercised and control animals. The intermittent program of RE was able to recover the behavioral, corticosterone and Aβ alterations to the CTRL levels. In addition, the RE protocol increased the number of microglial cells in the hippocampus of APP/PS1 mice. Despite these alterations, no memory impairment was observed in APP/PS1 mice in the novel object recognition test.
Discussion: Altogether, the present results suggest that RE plays a role in alleviating AD symptoms, and highlight the beneficial effects of RE training as a complementary treatment for AD.
Keywords: Alzheimer’s disease; amyloid-beta precursor protein; corticosterone; locomotor activity; resistance exercise.
Copyright © 2023 Campos, Ribeiro, Hashiguchi, Glaser, Milanis, Gimenes, Suchecki, Arida, Ulrich and Monteiro Longo.
Conflict of interest statement
HU is a scientific advisor of TissueGnostics (Vienna, Austria). The remaining authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Distinct Effects of the Hippocampal Transplantation of Neural and Mesenchymal Stem Cells in a Transgenic Model of Alzheimer's Disease.Stem Cell Rev Rep. 2022 Feb;18(2):781-791. doi: 10.1007/s12015-021-10321-9. Epub 2022 Jan 8. Stem Cell Rev Rep. 2022. PMID: 34997526
-
Resistance Exercise Decreases Amyloid Load and Modulates Inflammatory Responses in the APP/PS1 Mouse Model for Alzheimer's Disease.J Alzheimers Dis. 2020;73(4):1525-1539. doi: 10.3233/JAD-190729. J Alzheimers Dis. 2020. PMID: 31958083
-
Long-term running exercise improves cognitive function and promotes microglial glucose metabolism and morphological plasticity in the hippocampus of APP/PS1 mice.J Neuroinflammation. 2022 Feb 5;19(1):34. doi: 10.1186/s12974-022-02401-5. J Neuroinflammation. 2022. PMID: 35123512 Free PMC article.
-
Treadmill Exercise Promotes Microglial β-Amyloid Clearance and Prevents Cognitive Decline in APP/PS1 Mice.Neuroscience. 2022 May 21;491:122-133. doi: 10.1016/j.neuroscience.2022.03.043. Epub 2022 Apr 6. Neuroscience. 2022. PMID: 35398179
-
Exercise therapy to prevent and treat Alzheimer's disease.Front Aging Neurosci. 2023 Aug 4;15:1243869. doi: 10.3389/fnagi.2023.1243869. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37600508 Free PMC article. Review.
Cited by
-
Muscle Cathepsin B treatment improves behavioral and neurogenic deficits in a mouse model of Alzheimer's Disease.bioRxiv [Preprint]. 2025 Jan 22:2025.01.20.633414. doi: 10.1101/2025.01.20.633414. bioRxiv. 2025. PMID: 39896474 Free PMC article. Preprint.
-
An Interaction between Brain-Derived Neurotrophic Factor and Stress-Related Glucocorticoids in the Pathophysiology of Alzheimer's Disease.Int J Mol Sci. 2024 Jan 27;25(3):1596. doi: 10.3390/ijms25031596. Int J Mol Sci. 2024. PMID: 38338875 Free PMC article. Review.
-
Physical exercise regulates microglia in health and disease.Front Neurosci. 2024 Jun 7;18:1420322. doi: 10.3389/fnins.2024.1420322. eCollection 2024. Front Neurosci. 2024. PMID: 38911597 Free PMC article. Review.
-
Effectiveness of Resistance Exercise on Cognitive Function in Animal Models of Alzheimer Disease: A Systematic Review and Meta-Analysis.J Prev Alzheimers Dis. 2024;11(4):998-1012. doi: 10.14283/jpad.2024.75. J Prev Alzheimers Dis. 2024. PMID: 39044511 Free PMC article.
-
Research progress on resistance exercise therapy for improving cognitive function in patients with AD and muscle atrophy.Front Aging Neurosci. 2025 Apr 8;17:1552905. doi: 10.3389/fnagi.2025.1552905. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40271180 Free PMC article. Review.
References
-
- Alzheimer A. (1907). Uber eine eigenartige Erkrankung der Hirnrinde. Zentralbl. Nervenh. Psychol. 18, 177–179.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

