An atomically smooth container: Can the native oxide promote supercooling of liquid gallium?
- PMID: 37091232
- PMCID: PMC10113873
- DOI: 10.1016/j.isci.2023.106493
An atomically smooth container: Can the native oxide promote supercooling of liquid gallium?
Abstract
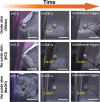
Metals tend to supercool-that is, they freeze at temperatures below their melting points. In general, supercooling is less favorable when liquids are in contact with nucleation sites such as rough surfaces. Interestingly, bulk gallium (Ga) can significantly supercool, even when it is in contact with heterogeneous surfaces that could provide nucleation sites. We hypothesized that the native oxide on Ga provides an atomically smooth interface that prevents Ga from directly contacting surfaces, and thereby promotes supercooling. Although many metals form surface oxides, Ga is a convenient metal for studying supercooling because its melting point of 29.8°C is near room temperature. Using differential scanning calorimetry (DSC), we show that freezing of Ga with the oxide occurs at a lower temperature (-15.6 ± 3.5°C) than without the oxide (6.9 ± 2.0°C when the oxide is removed by HCl). We also demonstrate that the oxide enhances supercooling via macroscopic observations of freezing. These findings explain why Ga supercools and have implications for emerging applications of Ga that rely on it staying in the liquid state.
Keywords: Inorganic materials; Materials chemistry; Materials science.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Sustainable Thermal Regulation of Electronics via Mitigated Supercooling of Porous Gallium-Based Phase Change Materials.Adv Sci (Weinh). 2024 Jun;11(23):e2310185. doi: 10.1002/advs.202310185. Epub 2024 Apr 18. Adv Sci (Weinh). 2024. PMID: 38634574 Free PMC article.
-
Cryo-scanning electron microscopic study on freezing behavior of xylem ray parenchyma cells in hardwood species.Micron. 2000 Dec;31(6):669-86. doi: 10.1016/s0968-4328(99)00103-1. Micron. 2000. PMID: 10838028
-
Intracellular ice formation in yeast cells vs. cooling rate: predictions from modeling vs. experimental observations by differential scanning calorimetry.Cryobiology. 2009 Apr;58(2):157-65. doi: 10.1016/j.cryobiol.2008.11.011. Epub 2008 Dec 11. Cryobiology. 2009. PMID: 19118541 Free PMC article.
-
Supercooling-Promoting (Anti-ice Nucleation) Substances.Adv Exp Med Biol. 2018;1081:289-320. doi: 10.1007/978-981-13-1244-1_16. Adv Exp Med Biol. 2018. PMID: 30288716 Review.
-
Harnessing the Rheological Properties of Liquid Metals To Shape Soft Electronic Conductors for Wearable Applications.Acc Chem Res. 2019 Mar 19;52(3):534-544. doi: 10.1021/acs.accounts.8b00489. Epub 2019 Feb 4. Acc Chem Res. 2019. PMID: 30714364 Review.
Cited by
-
Sustainable Thermal Regulation of Electronics via Mitigated Supercooling of Porous Gallium-Based Phase Change Materials.Adv Sci (Weinh). 2024 Jun;11(23):e2310185. doi: 10.1002/advs.202310185. Epub 2024 Apr 18. Adv Sci (Weinh). 2024. PMID: 38634574 Free PMC article.
-
Gallium-Carbon: A Universal Composite for Sustainable 3D Printing of Integrated Sensor-Heater-Battery Systems in Wearable and Recyclable Electronics.ACS Appl Mater Interfaces. 2024 Jun 26;16(25):32812-32823. doi: 10.1021/acsami.4c02706. Epub 2024 Jun 15. ACS Appl Mater Interfaces. 2024. PMID: 38878000 Free PMC article.
References
-
- Safari A., Saidur R., Sulaiman F.A., Xu Y., Dong J. A review on supercooling of phase change materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017;70:905–919.
-
- Paradis P.-F., Ishikawa T., Lee G.-W., Holland-Moritz D., Brillo J., Rhim W.-K., Okada J.T. Materials properties measurements and particle beam interactions studies using electrostatic levitation. Mater. Sci. Eng. R Rep. 2014;76:1–53.
-
- Fournier D'albe E.M. Some experiments on the condensation of water vapour at temperatures below 0. Q. J. R. Meteorol. Soc. 1949;75:1–16.
-
- Briggs L.J. Gallium: thermal conductivity; supercooling; negative pressure. J. Chem. Phys. 1957;26:784–786.
-
- Rodriguez S.E., Pings C.J. X-Ray diffraction studies of stable and supercooled liquid gallium. J. Chem. Phys. 1965;42:2435–2437.
LinkOut - more resources
Full Text Sources

