An inducible explant model of osteoclast-osteoprogenitor coordination in exacerbated osteoclastogenesis
- PMID: 37091244
- PMCID: PMC10119607
- DOI: 10.1016/j.isci.2023.106470
An inducible explant model of osteoclast-osteoprogenitor coordination in exacerbated osteoclastogenesis
Abstract
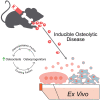
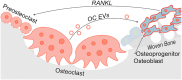
Elucidating a basic blueprint of osteoclast-osteoblast coordination in skeletal remodeling and understanding how this coordination breaks down with age and disease is essential for addressing the growing skeletal health problem in our aging population. The paucity of simple, activatable, biologically relevant models of osteoclast-osteoblast coordination has hindered our understanding of how skeletal remolding is regulated. Here, we describe an inducible ex vivo model of osteoclast-osteoblast progenitor coordination. Induction activates the release of osteoclastogenic factors from osteoprogenitors, which elicits the differentiation and fusion of neighboring preosteoclasts. In turn, multinucleated osteoclasts release soluble coupling factors, RANK+ extracellular vesicles and promote osteoprogenitor proliferation, recapitulating aspects of perturbed coordination in diseases underpinned by excessive osteoclast formation. We expect this model to expedite the investigation of cell-cell fusion, osteoclast-osteoblast progenitor coordination, and extracellular vesicle signaling during bone remodeling and offer a powerful tool for evaluating signaling cascades and novel therapeutic interventions in osteoclast-linked skeletal disease.
Keywords: Biological sciences; Cell biology; Stem cells research.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Integrative Single-Cell RNA-Seq and ATAC-Seq Identifies Transcriptional and Epigenetic Blueprint Guiding Osteoclastogenic Trajectory.J Bone Miner Res. 2025 Jun 19:zjaf084. doi: 10.1093/jbmr/zjaf084. Online ahead of print. J Bone Miner Res. 2025. PMID: 40577680
-
High mobility group box 1 protein regulates osteoclastogenesis through direct actions on osteocytes and osteoclasts in vitro.J Cell Biochem. 2019 Oct;120(10):16741-16749. doi: 10.1002/jcb.28932. Epub 2019 May 20. J Cell Biochem. 2019. PMID: 31106449 Free PMC article.
-
Human Infrapatellar Fat Pad Mesenchymal Stem Cell-derived Extracellular Vesicles Purified by Anion Exchange Chromatography Suppress Osteoarthritis Progression in a Mouse Model.Clin Orthop Relat Res. 2024 Jul 1;482(7):1246-1262. doi: 10.1097/CORR.0000000000003067. Epub 2024 Apr 19. Clin Orthop Relat Res. 2024. PMID: 38662932 Free PMC article.
-
Interventions for promoting habitual exercise in people living with and beyond cancer.Cochrane Database Syst Rev. 2018 Sep 19;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. Cochrane Database Syst Rev. 2018. PMID: 30229557 Free PMC article.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
Cited by
-
RANKL inhibition reduces lesional cellularity and Gαs variant expression and enables osteogenic maturation in fibrous dysplasia.Bone Res. 2024 Feb 20;12(1):10. doi: 10.1038/s41413-023-00311-7. Bone Res. 2024. PMID: 38378678 Free PMC article. Clinical Trial.
References
Grants and funding
LinkOut - more resources
Full Text Sources

