Combined neuromuscular electrical stimulation and transcutaneous spinal direct current stimulation increases motor cortical plasticity in healthy humans
- PMID: 37091256
- PMCID: PMC10115158
- DOI: 10.3389/fnins.2022.1034451
Combined neuromuscular electrical stimulation and transcutaneous spinal direct current stimulation increases motor cortical plasticity in healthy humans
Abstract
Introduction: Neuromuscular electrical stimulation (NMES) induces neural plasticity of the central nervous system (CNS) and improves motor function in patients with CNS lesions. However, the extended stimulus duration of NMES reduces its clinical applicability. Transcutaneous spinal direct current stimulation (tsDCS), which increases afferent input, may enhance the effects and reduce the stimulus duration of NMES. This study investigated the excitability of the motor cortex, somatosensory cortex, and spinal motor neurons after the combined stimulation of NMES and tsDCS.
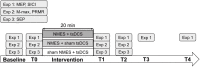
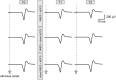
Methods: Among the 55 participants in this study, 24 were allocated to experiment 1, 15 to experiment 2, and 16 to experiment 3. They received intervention for 20 min on different days: (1) NMES combined with tsDCS (NMES + tsDCS), (2) NMES combined with sham tsDCS (NMES + sham tsDCS), and (3) sham NMES combined with tsDCS (sham NMES + tsDCS). NMES was delivered to the right common peroneal nerve at 25 Hz with the intensity at 120% of the motor threshold. For tsDCS, the cathodal electrode was positioned on the thoracic 10th-12th vertebral levels, and the anodal electrode was located on the right shoulder. The stimulus intensity was 2.5 mA. In experiment 1, motor evoked potentials (MEPs) and short-latency intracortical inhibition (SICI) were measured by transcranial magnetic stimulation up to 60 min after stimulation. The spinal motor neurons' excitability was assessed by recording the posterior root muscle reflex (PRMR) induced via transcutaneous spinal cord stimulation in experiment 2, and the primary somatosensory cortex excitability was evaluated by recording the somatosensory evoked potentials (SEPs) in experiment 3 up to 15 min after stimulation.
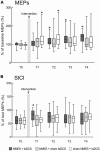
Results: Compared to before the stimulation, NMES + tsDCS significantly increased MEP for 60 min or more, and significantly decreased SICI immediately after. Conversely contrast, the PRMR significantly decreased immediately after, and SEPs were unchanged.
Discussion: These results suggest that simultaneous afferent inputs from different stimulus positions critically induce primary motor cortex plasticity. The combined stimulation of NMES with tsDCS may facilitate the development of a new neurorehabilitation technique.
Keywords: afferent input; corticospinal projection; motor cortical excitability; neural plasticity; spinal excitability.
Copyright © 2023 Koseki, Kudo, Yoshida, Nito, Takano, Jin, Tanabe, Sato, Katoh and Yamaguchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
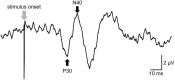
Similar articles
-
Transcutaneous spinal direct current stimulation modulates human corticospinal system excitability.J Neurophysiol. 2015 Jul;114(1):440-6. doi: 10.1152/jn.00490.2014. Epub 2015 Apr 29. J Neurophysiol. 2015. PMID: 25925328 Free PMC article.
-
Spinal Direct Current Stimulation Modulates Short Intracortical Inhibition.Neuromodulation. 2015 Dec;18(8):686-93. doi: 10.1111/ner.12298. Epub 2015 Apr 16. Neuromodulation. 2015. PMID: 25880098
-
Transcutaneous spinal direct current stimulation increases corticospinal transmission and enhances voluntary motor output in humans.Physiol Rep. 2020 Aug;8(16):e14531. doi: 10.14814/phy2.14531. Physiol Rep. 2020. PMID: 32812363 Free PMC article.
-
What Else Can Be Done by the Spinal Cord? A Review on the Effectiveness of Transpinal Direct Current Stimulation (tsDCS) in Stroke Recovery.Int J Mol Sci. 2023 Jun 15;24(12):10173. doi: 10.3390/ijms241210173. Int J Mol Sci. 2023. PMID: 37373323 Free PMC article. Review.
-
Modulations in neural pathways excitability post transcutaneous spinal cord stimulation among individuals with spinal cord injury: a systematic review.Front Neurosci. 2024 Mar 25;18:1372222. doi: 10.3389/fnins.2024.1372222. eCollection 2024. Front Neurosci. 2024. PMID: 38591069 Free PMC article.
References
-
- Andrews R. K., Schabrun S. M., Ridding M. C., Galea M. P., Hodges P. W., Chipchase L. S. (2013). The effect of electrical stimulation on corticospinal excitability is dependent on application duration: A same subject pre-post test design. J. Neuroeng. Rehabil. 10:51. 10.1186/1743-0003-10-51 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

