Long-Term Safety and Efficacy of Recombinant Human Parathyroid Hormone (1-84) in Adults With Chronic Hypoparathyroidism
- PMID: 37091306
- PMCID: PMC10119703
- DOI: 10.1210/jendso/bvad043
Long-Term Safety and Efficacy of Recombinant Human Parathyroid Hormone (1-84) in Adults With Chronic Hypoparathyroidism
Abstract
Context: Chronic hypoparathyroidism is conventionally treated with oral calcium and active vitamin D to reach and maintain targeted serum calcium and phosphorus levels, but some patients remain inadequately controlled.
Objective: To assess long-term safety and efficacy of recombinant human parathyroid hormone (1-84) (rhPTH(1-84)) treatment.
Methods: This was an open-label extension study at 12 US centers. Adults (n = 49) with chronic hypoparathyroidism were included. The intervention was rhPTH(1-84) for 6 years. The main outcome measures were safety, biochemical measures, oral supplement doses, bone indices.
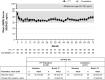
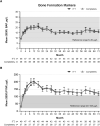
Results: Thirty-eight patients (77.6%) completed the study. Throughout 72 months, mean albumin-adjusted serum calcium was within 2.00 to 2.25 mmol/L (8.0-9.0 mg/dL). At baseline, 65% of patients with measurements (n = 24/37) were hypercalciuric; of these, 54% (n = 13/24) were normocalciuric at month 72. Mean serum phosphorus declined from 1.6 ± 0.19 mmol/L at baseline (n = 49) to 1.3 ± 0.20 mmol/L at month 72 (n = 36). Mean estimated glomerular filtration rate was stable. rhPTH(1-84)-related adverse events were reported in 51.0% of patients (n = 25/49); all but 1 event were mild/moderate in severity. Mean oral calcium supplementation reduced by 45% ± 113.6% and calcitriol by 74% ± 39.3%. Bone turnover markers declined by month 32 to a plateau above pretreatment values; only aminoterminal propeptide of type 1 collagen remained outside the reference range. Mean bone mineral density z score fell at one-third radius and was stable at other sites.
Conclusion: 6 years of rhPTH(1-84) treatment was associated with sustained improvements in biochemical parameters, a reduction in the percentage of patients with hypercalciuria, stable renal function, and decreased supplement requirements. rhPTH(1-84) was well tolerated; no new safety signals were identified.
Keywords: active vitamin D; bone turnover; calcium; hypoparathyroidism; mineral homeostasis; recombinant human parathyroid hormone (1-84).
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures





Similar articles
-
Safety and Efficacy of 5 Years of Treatment With Recombinant Human Parathyroid Hormone in Adults With Hypoparathyroidism.J Clin Endocrinol Metab. 2019 Nov 1;104(11):5136-5147. doi: 10.1210/jc.2019-01010. J Clin Endocrinol Metab. 2019. PMID: 31369089 Free PMC article.
-
Therapy of Hypoparathyroidism With rhPTH(1-84): A Prospective, 8-Year Investigation of Efficacy and Safety.J Clin Endocrinol Metab. 2019 Nov 1;104(11):5601-5610. doi: 10.1210/jc.2019-00893. J Clin Endocrinol Metab. 2019. PMID: 31310310 Free PMC article.
-
AN OPEN-LABEL EXTENSION STUDY OF PARATHYROID HORMONE RHPTH(1-84) IN ADULTS WITH HYPOPARATHYROIDISM.Endocr Pract. 2016 May;22(5):523-32. doi: 10.4158/EP15936.OR. Epub 2015 Dec 18. Endocr Pract. 2016. PMID: 26684150 Clinical Trial.
-
Drug safety evaluation of parathyroid hormone for hypocalcemia in patients with hypoparathyroidism.Expert Opin Drug Saf. 2017 May;16(5):617-625. doi: 10.1080/14740338.2017.1311322. Epub 2017 Apr 3. Expert Opin Drug Saf. 2017. PMID: 28332412 Review.
-
Recombinant Human Parathyroid Hormone (1-84): A Review in Hypoparathyroidism.Drugs. 2015 Jul;75(11):1293-303. doi: 10.1007/s40265-015-0438-2. Drugs. 2015. PMID: 26177893 Review.
Cited by
-
Preclinical development of EXT608, an investigational parathyroid hormone derivative with extended half-life for the treatment of hypoparathyroidism.JBMR Plus. 2024 Apr 18;8(6):ziae045. doi: 10.1093/jbmrpl/ziae045. eCollection 2024 Jun. JBMR Plus. 2024. PMID: 38721043 Free PMC article.
-
New insights into the vitamin D/PTH axis in endocrine-driven metabolic bone diseases.Endocrine. 2024 Sep;85(3):1007-1019. doi: 10.1007/s12020-024-03784-6. Epub 2024 Apr 17. Endocrine. 2024. PMID: 38632163 Review.
-
Switch From rhPTH1-84 to TransCon PTH With Individual Dose Adjustment in Adult Hypoparathyroidism-4-Week Results.J Endocr Soc. 2025 Jun 30;9(9):bvaf113. doi: 10.1210/jendso/bvaf113. eCollection 2025 Sep. J Endocr Soc. 2025. PMID: 40709359 Free PMC article.
References
-
- Shoback DM, Bilezikian JP, Costa AG, et al. . Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab. 2016;101(6):2300‐2312. - PubMed
-
- Bollerslev J, Rejnmark L, Marcocci C, et al. . European Society of Endocrinology clinical guideline: treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173(2):G1‐G120. - PubMed
-
- Brandi ML, Bilezikian JP, Shoback D, et al. . Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab. 2016;101(6):2273‐2283. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
