Transcranial focused ultrasound selectively increases perfusion and modulates functional connectivity of deep brain regions in humans
- PMID: 37091318
- PMCID: PMC10114286
- DOI: 10.3389/fncir.2023.1120410
Transcranial focused ultrasound selectively increases perfusion and modulates functional connectivity of deep brain regions in humans
Abstract
Background: Low intensity, transcranial focused ultrasound (tFUS) is a re-emerging brain stimulation technique with the unique capability of reaching deep brain structures non-invasively.
Objective/hypothesis: We sought to demonstrate that tFUS can selectively and accurately target and modulate deep brain structures in humans important for emotional functioning as well as learning and memory. We hypothesized that tFUS would result in significant longitudinal changes in perfusion in the targeted brain region as well as selective modulation of BOLD activity and BOLD-based functional connectivity of the target region.
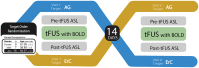
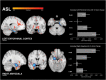
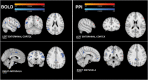
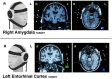
Methods: In this study, we collected MRI before, simultaneously during, and after tFUS of two deep brain structures on different days in sixteen healthy adults each serving as their own control. Using longitudinal arterial spin labeling (ASL) MRI and simultaneous blood oxygen level dependent (BOLD) functional MRI, we found changes in cerebral perfusion, regional brain activity and functional connectivity specific to the targeted regions of the amygdala and entorhinal cortex (ErC).
Results: tFUS selectively increased perfusion in the targeted brain region and not in the contralateral homolog or either bilateral control region. Additionally, tFUS directly affected BOLD activity in a target specific fashion without engaging auditory cortex in any analysis. Finally, tFUS resulted in selective modulation of the targeted functional network connectivity.
Conclusion: We demonstrate that tFUS can selectively modulate perfusion, neural activity and connectivity in deep brain structures and connected networks. Lack of auditory cortex findings suggests that the mechanism of tFUS action is not due to auditory or acoustic startle response but rather a direct neuromodulatory process. Our findings suggest that tFUS has the potential for future application as a novel therapy in a wide range of neurological and psychiatric disorders associated with subcortical pathology.
Keywords: amygdala; brain perfusion; entorhina cortex; functional connectivity; transcranial focused ultrasound.
Copyright © 2023 Kuhn, Spivak, Dang, Becerra, Halavi, Rotstein, Rosenberg, Hiller, Swenson, Cvijanovic, Dang, Sun, Kronemyer, Berlow, Revett, Suthana, Monti and Bookheimer.
Conflict of interest statement
NSu was a consultant for BrainSonix Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Transcranial Focused Ultrasound Targeting the Amygdala May Increase Psychophysiological and Subjective Negative Emotional Reactivity in Healthy Older Adults.Biol Psychiatry Glob Open Sci. 2024 Jun 5;4(5):100342. doi: 10.1016/j.bpsgos.2024.100342. eCollection 2024 Sep. Biol Psychiatry Glob Open Sci. 2024. PMID: 39092138 Free PMC article.
-
Alterations in large-scale resting-state network nodes following transcranial focused ultrasound of deep brain structures.Front Hum Neurosci. 2024 Dec 4;18:1486770. doi: 10.3389/fnhum.2024.1486770. eCollection 2024. Front Hum Neurosci. 2024. PMID: 39698148 Free PMC article.
-
Transcranial focused ultrasound of the amygdala modulates fear network activation and connectivity.Brain Stimul. 2024 Mar-Apr;17(2):312-320. doi: 10.1016/j.brs.2024.03.004. Epub 2024 Mar 4. Brain Stimul. 2024. PMID: 38447773 Clinical Trial.
-
Safety of transcranial focused ultrasound stimulation: A systematic review of the state of knowledge from both human and animal studies.Brain Stimul. 2019 Nov-Dec;12(6):1367-1380. doi: 10.1016/j.brs.2019.07.024. Epub 2019 Jul 31. Brain Stimul. 2019. PMID: 31401074
-
Transcranial Focused Ultrasound Neuromodulation in Psychiatry: Main Characteristics, Current Evidence, and Future Directions.Brain Sci. 2024 Oct 30;14(11):1095. doi: 10.3390/brainsci14111095. Brain Sci. 2024. PMID: 39595858 Free PMC article. Review.
Cited by
-
Cerebral hyperactivation across the Alzheimer's disease pathological cascade.Brain Commun. 2024 Oct 25;6(6):fcae376. doi: 10.1093/braincomms/fcae376. eCollection 2024. Brain Commun. 2024. PMID: 39513091 Free PMC article. Review.
-
Dynamic changes in human brain connectivity following ultrasound neuromodulation.Sci Rep. 2024 Dec 3;14(1):30025. doi: 10.1038/s41598-024-81102-w. Sci Rep. 2024. PMID: 39627315 Free PMC article.
-
Transcranial Focused Ultrasound Targeting the Amygdala May Increase Psychophysiological and Subjective Negative Emotional Reactivity in Healthy Older Adults.Biol Psychiatry Glob Open Sci. 2024 Jun 5;4(5):100342. doi: 10.1016/j.bpsgos.2024.100342. eCollection 2024 Sep. Biol Psychiatry Glob Open Sci. 2024. PMID: 39092138 Free PMC article.
-
The therapeutic potential of low-intensity focused ultrasound for treating substance use disorder.Front Psychiatry. 2024 Nov 19;15:1466506. doi: 10.3389/fpsyt.2024.1466506. eCollection 2024. Front Psychiatry. 2024. PMID: 39628494 Free PMC article. Review.
-
Low-intensity transcranial focused ultrasound of the amygdala modulates neural activation during emotion processing.Front Neuroimaging. 2025 May 30;4:1580623. doi: 10.3389/fnimg.2025.1580623. eCollection 2025. Front Neuroimaging. 2025. PMID: 40519563 Free PMC article.

