Biomaterial-assisted tumor therapy: A brief review of hydroxyapatite nanoparticles and its composites used in bone tumors therapy
- PMID: 37091350
- PMCID: PMC10119417
- DOI: 10.3389/fbioe.2023.1167474
Biomaterial-assisted tumor therapy: A brief review of hydroxyapatite nanoparticles and its composites used in bone tumors therapy
Abstract
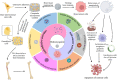
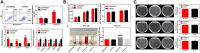
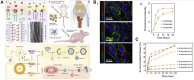
Malignant bone tumors can inflict significant damage to affected bones, leaving patients to contend with issues like residual tumor cells, bone defects, and bacterial infections post-surgery. However, hydroxyapatite nanoparticles (nHAp), the principal inorganic constituent of natural bone, possess numerous advantages such as high biocompatibility, bone conduction ability, and a large surface area. Moreover, nHAp's nanoscale particle size enables it to impede the growth of various tumor cells via diverse pathways. This article presents a comprehensive review of relevant literature spanning the past 2 decades concerning nHAp and bone tumors. The primary goal is to explore the mechanisms responsible for nHAp's ability to hinder tumor initiation and progression, as well as to investigate the potential of integrating other drugs and components for bone tumor diagnosis and treatment. Lastly, the article discusses future prospects for the development of hydroxyapatite materials as a promising modality for tumor therapy.
Keywords: MFH; PTT; bone tumor therapy; drug delivery; hydroxyapatite nanoparticles; tumor cell apoptosis.
Copyright © 2023 Zhang, Qiang, Liu, Fan, Si and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Controlled nanoparticle synthesis of Ag/Fe co-doped hydroxyapatite system for cancer cell treatment.Mater Sci Eng C Mater Biol Appl. 2019 May;98:311-323. doi: 10.1016/j.msec.2018.12.148. Epub 2019 Jan 3. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30813033
-
Characterization of natural hydroxyapatite originated from fish bone and its biocompatibility with osteoblasts.Mater Sci Eng C Mater Biol Appl. 2018 Sep 1;90:706-712. doi: 10.1016/j.msec.2018.04.026. Epub 2018 Apr 14. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 29853142
-
Nanoscale hydroxyapatite particles for bone tissue engineering.Acta Biomater. 2011 Jul;7(7):2769-81. doi: 10.1016/j.actbio.2011.03.019. Epub 2011 Apr 1. Acta Biomater. 2011. PMID: 21440094 Review.
-
Preparation and properties of a highly dispersed nano-hydroxyapatite colloid used as a reinforcing filler for chitosan.Mater Sci Eng C Mater Biol Appl. 2020 May;110:110689. doi: 10.1016/j.msec.2020.110689. Epub 2020 Jan 23. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32204004
-
Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration.Mater Sci Eng C Mater Biol Appl. 2020 May;110:110698. doi: 10.1016/j.msec.2020.110698. Epub 2020 Jan 29. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32204012 Free PMC article. Review.
Cited by
-
Microwave-Assisted Hydrothermal Treatment of Multifunctional Substituted Hydroxyapatite with Prospective Applications in Bone Regeneration.J Funct Biomater. 2023 Jul 19;14(7):378. doi: 10.3390/jfb14070378. J Funct Biomater. 2023. PMID: 37504872 Free PMC article.
-
Advancements in Photothermal Therapy Using Near-Infrared Light for Bone Tumors.Int J Mol Sci. 2024 Apr 9;25(8):4139. doi: 10.3390/ijms25084139. Int J Mol Sci. 2024. PMID: 38673726 Free PMC article. Review.
-
Application of Hydroxyapatite Obtained by Different Techniques: Metabolism and Microarchitecture Characteristics (Review).Sovrem Tekhnologii Med. 2024;16(6):60-75. doi: 10.17691/stm2024.16.6.06. Epub 2024 Dec 27. Sovrem Tekhnologii Med. 2024. PMID: 39896152 Free PMC article. Review.
References
-
- Abbasi Aval N., Pirayesh Islamian J., Hatamian M., Arabfirouzjaei M., Javadpour J., Rashidi M. R. (2016). Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment. Int. J. Pharm. 509 (1), 159–167. 10.1016/j.ijpharm.2016.05.046 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

