Synthesis of Carlactone Derivatives to Develop a Novel Inhibitor of Strigolactone Biosynthesis
- PMID: 37091382
- PMCID: PMC10116532
- DOI: 10.1021/acsomega.3c00098
Synthesis of Carlactone Derivatives to Develop a Novel Inhibitor of Strigolactone Biosynthesis
Abstract
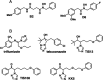
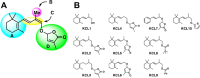
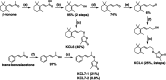
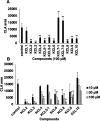
Strigolactones (SLs), phytohormones that inhibit shoot branching in plants, promote the germination of root-parasitic plants, such as Striga spp. and Orobanche spp., which drastically reduces the crop yield. Therefore, reducing SL production via chemical treatment may increase the crop yield. To design specific inhibitors, it is valid to utilize the substrate structure of the target proteins as lead compounds. In this study, we focused on Os900, a rice enzyme that oxidizes the SL precursor carlactone (CL) to 4-deoxyorobanchol (4DO), and synthesized 10 CL derivatives. The effects of the synthesized CL derivatives on SL biosynthesis were evaluated by the Os900 enzyme assay in vitro and by measuring 4DO levels in rice root exudates. We identified some CL derivatives that inhibited SL biosynthesis in vitro and in vivo.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Triflumizole as a Novel Lead Compound for Strigolactone Biosynthesis Inhibitor.Molecules. 2020 Nov 25;25(23):5525. doi: 10.3390/molecules25235525. Molecules. 2020. PMID: 33255720 Free PMC article.
-
Disruption of the rice 4-DEOXYOROBANCHOL HYDROXYLASE unravels specific functions of canonical strigolactones.Proc Natl Acad Sci U S A. 2023 Oct 17;120(42):e2306263120. doi: 10.1073/pnas.2306263120. Epub 2023 Oct 11. Proc Natl Acad Sci U S A. 2023. PMID: 37819983 Free PMC article.
-
Insect growth regulators with hydrazide moiety inhibit strigolactone biosynthesis in rice.J Pestic Sci. 2022 Feb 20;47(1):43-46. doi: 10.1584/jpestics.D21-063. J Pestic Sci. 2022. PMID: 35414758 Free PMC article.
-
Structure Elucidation and Biosynthesis of Orobanchol.Front Plant Sci. 2022 Feb 9;13:835160. doi: 10.3389/fpls.2022.835160. eCollection 2022. Front Plant Sci. 2022. PMID: 35222492 Free PMC article. Review.
-
Target sites for chemical regulation of strigolactone signaling.Front Plant Sci. 2014 Nov 5;5:623. doi: 10.3389/fpls.2014.00623. eCollection 2014. Front Plant Sci. 2014. PMID: 25414720 Free PMC article. Review.
References
-
- Ejeta G. Breeding for Striga Resistance in Sorghum: Exploitation of an Intricate Host–Parasite Biology. Crop Sci. 2007, 47, S216–S227. 10.2135/cropsci2007.04.0011IPBS. - DOI
-
- Butler L. G.“Allelopathy, Organisms, Processes and Applications”; Inderjit K. M., Dakshini M.; Enhelling F. A., Eds.; American Chemical Society. 1995; pp 158–166.
LinkOut - more resources
Full Text Sources
Miscellaneous

