Microwave-Assisted Synthesis, Structure, and Preliminary Biological Evaluation of Novel 6-Methoxy-5,6-dihydro-5-azapurines
- PMID: 37091407
- PMCID: PMC10116508
- DOI: 10.1021/acsomega.3c00765
Microwave-Assisted Synthesis, Structure, and Preliminary Biological Evaluation of Novel 6-Methoxy-5,6-dihydro-5-azapurines
Abstract
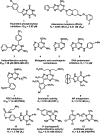
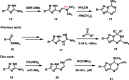
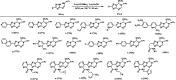
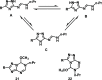
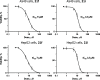
We herein disclose the microwave-assisted synthesis of previously unreported 6-methoxy-5,6-dihydro-5-azapurines, whose purine-like scaffold is promising for drug discovery. The method is simple, fast, and relies on easily accessible reagents such as trimethyl orthoformate, acetic acid, and aminotriazole-derived N,N'-disubstituted formamidines. The preliminary biological evaluation revealed that selected representatives of synthesized 6-methoxy-5,6-dihydro-5-azapurines dose-dependently reduce the viability of HepG2 and A549 cancer cells having little to no influence on five tested purinergic receptors.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Three-component microwave-assisted synthesis of 3,5-disubstituted pyrazolo[3,4-d]pyrimidin-4-ones.RSC Adv. 2022 Mar 16;12(14):8323-8332. doi: 10.1039/d2ra00980c. eCollection 2022 Mar 15. RSC Adv. 2022. PMID: 35424837 Free PMC article.
-
Microwave-Assisted Synthesis and Anticancer Activity of Triazolyl Thiazolidine Derivatives of Pyrene.Acta Chim Slov. 2019 Sep;66(3):700-710. Acta Chim Slov. 2019. PMID: 33855518
-
Solution-phase microwave assisted parallel synthesis of N,N'-disubstituted thioureas derived from benzoic acid: biological evaluation and molecular docking studies.Eur J Med Chem. 2013;70:487-96. doi: 10.1016/j.ejmech.2013.10.012. Epub 2013 Oct 12. Eur J Med Chem. 2013. PMID: 24185379
-
8-Azapurines as isosteric purine fluorescent probes for nucleic acid and enzymatic research.Mol Biosyst. 2014 Nov;10(11):2756-74. doi: 10.1039/c4mb00233d. Mol Biosyst. 2014. PMID: 25124808 Review.
-
8-azapurine nucleus: a versatile scaffold for different targets.Mini Rev Med Chem. 2009 Oct;9(12):1367-78. doi: 10.2174/138955709789957440. Mini Rev Med Chem. 2009. PMID: 19939217 Review.
Cited by
-
Pyrazinyl-Substituted Aminoazoles as Covalent Inhibitors of Thrombin: Synthesis, Structure, and Anticoagulant Properties.ACS Pharmacol Transl Sci. 2024 Dec 11;8(1):146-172. doi: 10.1021/acsptsci.4c00515. eCollection 2025 Jan 10. ACS Pharmacol Transl Sci. 2024. PMID: 39816788
References
-
- Ganeshpurkar A.; Gutti G.; Singh S. K.. RNA-dependent RNA polymerases and their emerging roles in antiviral therapy. In Viral Polymerases; Gupta S. P., Eds.; Elsevier, 2019; pp 1–42.
-
- Chauhan M.; Kumar R. A comprehensive review on bioactive fused heterocycles as purine-utilizing enzymes inhibitors. Med. Chem. Res. 2015, 24 (6), 2259–2282. 10.1007/s00044-014-1295-3. - DOI
-
- Bera H.; Tan B. J.; Sun L.; Dolzhenko A. V.; Chui W. K.; Chiu G. N. A structure-activity relationship study of 1,2,4-triazolo[1,5-a][1,3,5]triazin-5,7-dione and its 5-thioxo analogues on anti-thymidine phosphorylase and associated anti-angiogenic activities. Eur. J. Med. Chem. 2013, 67, 325–34. 10.1016/j.ejmech.2013.06.051. - DOI - PubMed
-
- Vu C. B.; Pan D.; Peng B.; Kumaravel G.; Smits G.; Jin X.; Phadke D.; Engber T.; Huang C.; Reilly J.; Tam S.; Grant D.; Hetu G.; Petter R. C. Novel diamino derivatives of [1,2,4]triazolo[1,5-a][1,3,5]triazine as potent and selective adenosine A2a receptor antagonists. J. Med. Chem. 2005, 48 (6), 2009–18. 10.1021/jm0498396. - DOI - PubMed
LinkOut - more resources
Full Text Sources

