Near-infrared fluorescence angiography with indocyanine green for perfusion assessment of DIEP and msTRAM flaps: A Dutch multicenter randomized controlled trial
- PMID: 37091505
- PMCID: PMC10119502
- DOI: 10.1016/j.conctc.2023.101128
Near-infrared fluorescence angiography with indocyanine green for perfusion assessment of DIEP and msTRAM flaps: A Dutch multicenter randomized controlled trial
Abstract
Background: A common complication after a DIEP flap reconstruction is the occurrence of fat necrosis due to inadequate flap perfusion zones. Intraoperative identification of ischemic zones in the DIEP flap could be optimized using indocyanine green near-infrared fluorescence angiography (ICG-NIR-FA). This randomized controlled trial aims to determine whether intraoperative ICG-NIR-FA for the assessment of DIEP flap perfusion decreases the occurrence of fat necrosis.
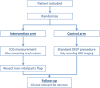
Design/methods: This article describes the protocol of a Dutch multicenter randomized controlled clinical trial: the FAFI-trial. Females who are electively scheduled for autologous breast reconstruction using DIEP or muscle-sparing transverse rectus abdominis muscle (msTRAM) flaps are included. A total of 280 patients will be included in a 1:1 ratio between both study arms. In the intervention arm, the intraoperative assessment of flap perfusion will be based on both regular clinical parameters and ICG-NIR-FA. The control arm consists of flap perfusion evaluation only through the regular clinical parameters, while ICG-NIR-FA images are obtained during surgery for which the surgeon is blinded. The main study endpoint is the difference in percentage of clinically relevant fat necrosis between both study arms, evaluated two weeks and three months after reconstruction.
Conclusion: The FAFI-trial, a Dutch multicenter randomized controlled clinical trial, aims to investigate the clinical added value of intraoperative use of standardized ICG-NIR-FA for assessment of DIEP/msTRAM flap perfusion in the reduction of fat necrosis.
Clinical trial registration number: NCT05507710; NL 68623.058.18.
Keywords: Breast reconstruction; Fat necrosis; Free flap surgery; Indocyanine green; Near-infrared fluorescence; Perfusion; Quantification; Reconstructive surgery.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Healy C., Allen R.J., Sr The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J. Reconstr. Microsurg. 2014;30(2):121–125. - PubMed
-
- Patel N.G., Ramakrishnan V. Microsurgical tissue transfer in breast reconstruction. Clin. Plast. Surg. 2017;44(2):345–359. - PubMed
-
- Hauck T., Horch R.E., Schmitz M., et al. Secondary breast reconstruction after mastectomy using the DIEP flap. Surg Oncol. 2018;27(3):513. - PubMed
-
- Kouwenberg C.A.E., de Ligt K.M., Kranenburg L.W., et al. Long-term health-related quality of Life after four common surgical treatment options for breast cancer and the effect of complications: a retrospective patient-reported survey among 1871 patients. Plast. Reconstr. Surg. 2020;146(1):1–13. - PubMed
-
- Damen T.H., Timman R., Kunst E.H., et al. High satisfaction rates in women after DIEP flap breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2010;63(1):93–100. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous