Normative mice retinal thickness: 16-month longitudinal characterization of wild-type mice and changes in a model of Alzheimer's disease
- PMID: 37091517
- PMCID: PMC10117679
- DOI: 10.3389/fnagi.2023.1161847
Normative mice retinal thickness: 16-month longitudinal characterization of wild-type mice and changes in a model of Alzheimer's disease
Erratum in
-
Corrigendum: Normative mice retinal thickness: 16-month longitudinal characterization of wild-type mice and changes in a model of Alzheimer's disease.Front Aging Neurosci. 2024 May 10;16:1423913. doi: 10.3389/fnagi.2024.1423913. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38800612 Free PMC article.
Abstract
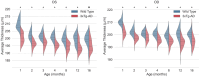
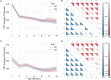
Animal models of disease are paramount to understand retinal development, the pathophysiology of eye diseases, and to study neurodegeneration using optical coherence tomography (OCT) data. In this study, we present a comprehensive normative database of retinal thickness in C57BL6/129S mice using spectral-domain OCT data. The database covers a longitudinal period of 16 months, from 1 to 16 months of age, and provides valuable insights into retinal development and changes over time. Our findings reveal that total retinal thickness decreases with age, while the thickness of individual retinal layers and layer aggregates changes in different ways. For example, the outer plexiform layer (OPL), photoreceptor inner segments (ILS), and retinal pigment epithelium (RPE) thickened over time, whereas other retinal layers and layer aggregates became thinner. Additionally, we compare the retinal thickness of wild-type (WT) mice with an animal model of Alzheimer's disease (3 × Tg-AD) and show that the transgenic mice exhibit a decrease in total retinal thickness compared to age-matched WT mice, with statistically significant differences observed at all evaluated ages. This normative database of retinal thickness in mice will serve as a reference for future studies on retinal changes in neurodegenerative and eye diseases and will further our understanding of the pathophysiology of these conditions.
Keywords: 3 × Tg-AD animal model; Alzheimer's disease; normative data; optical coherence tomography; retinal thickness.
Copyright © 2023 Batista, Guimarães, Martins, Moreira, Ambrósio, Castelo-Branco, Serranho and Bernardes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Berger A., Cavallero S., Dominguez E., Barbe P., Simonutti M., Sahel J.-A., et al. . (2014). Spectral-domain optical coherence tomography of the rodent eye: highlighting layers of the outer retina using signal averaging and comparison with histology. PLoS ONE 9, e96494. 10.1371/journal.pone.0096494 - DOI - PMC - PubMed
-
- Bernardes R., Ferreira H., Guimarães P., Serranho P. (2022). “Shedding light on early central nervous system changes for alzheimer's disease through the retina: an animal study,” in Proceedings of the 2nd International Conference on Image Processing and Vision Engineering (SCITEPRESS - Science and Technology Publications) (Prague: ), 247−258. 10.5220/0011125600003209 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

