Magneto-structural maps and bridged-ligand effect for dichloro-bridged dinuclear copper(ii) complexes: a theoretical perspective
- PMID: 37091610
- PMCID: PMC10116190
- DOI: 10.1039/d3ra00585b
Magneto-structural maps and bridged-ligand effect for dichloro-bridged dinuclear copper(ii) complexes: a theoretical perspective
Abstract
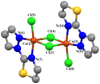
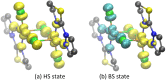
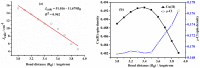
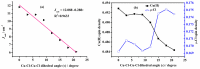
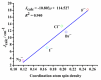
Theoretical understanding of magneto-structural correlations in dichloro-bridged dicopper(ii) complexes can guide the design of magnetic materials having broad-scale applications. However, previous reports suggest these correlations are complicated and unclear. To clarify possible correlations, magnetic coupling constants (J calc) of variants of a representative {Cu-(μ-Cl)2-Cu} complex A were calculated through BS-DFT. The variation of the Cu-(μ-Cl)-Cu angle (α), Cu⋯Cu distance (R 0), and Cu-Cl-Cu-Cl dihedral angle (τ) followed by structural optimization and calculation of the magnetic coupling constant (J calc) revealed several trends. J calc increased linearly with R 0 and τ, and initially increased and then decreased with α. Further, bridging ligand effects on J calc for dicopper(ii) complexes were evaluated through BS-DFT; the results revealed that J calc increased with increasing ligand field strength (I- < Br- < Cl- < N3 - < F-). Furthermore, a linear relationship was found between the spin density of the bridging ligand and J calc.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Anion influence on the structure and magnetic properties of a series of multidimensional pyrimidine-2-carboxylato-bridged copper(II) complexes.Inorg Chem. 2008 Sep 15;47(18):8143-58. doi: 10.1021/ic800625w. Epub 2008 Aug 13. Inorg Chem. 2008. PMID: 18698761
-
Synthesis, crystal structures and magnetic properties of bis(μ-dialkoxo)-bridged linear trinuclear copper(II) complexes with aminoalcohol ligands: a theoretical/experimental magneto-structural study.Dalton Trans. 2012 Mar 7;41(9):2648-58. doi: 10.1039/c2dt11628f. Epub 2012 Jan 11. Dalton Trans. 2012. PMID: 22234645
-
Framework solids based on copper(II) halides (Cl/Br) and methylene-bridged bis(1-hydroxybenzotriazole): synthesis, crystal structures, magneto-structural correlation, and density functional theory (DFT) studies.Inorg Chem. 2012 Oct 1;51(19):10148-57. doi: 10.1021/ic300629v. Epub 2012 Sep 13. Inorg Chem. 2012. PMID: 22974283
-
Electron/atom transfer in halo-bridged homobimetallic complexes. structure and donor-acceptor properties of face-to-face dicopper complexes with teraazamacrocyclic ligands.Inorg Chem. 2001 Mar 26;40(7):1614-25. doi: 10.1021/ic000726w. Inorg Chem. 2001. PMID: 11261972
-
Dinuclear Metallacycles with Single M-X-M Bridges (X = Cl-, Br-; M = Fe(II), Co(II), Ni(II), Cu(II), Zn(II), Cd(II)): Strong Antiferromagnetic Superexchange Interactions.Inorg Chem. 2017 Mar 6;56(5):2884-2901. doi: 10.1021/acs.inorgchem.6b02933. Epub 2017 Feb 20. Inorg Chem. 2017. PMID: 28218526
References
-
- Koval I. A. Gamez P. Belle C. Selmeczi K. Reedijk J. Chem. Soc. Rev. 2006;35:814–840. - PubMed
-
- Moore G. R. Huang Z. X. Eley C. G. S. Barker H. A. Williams G. Robinson M. N. Williams R. J. P. Faraday Discuss. Chem. Soc. 1982;74:311–329. - PubMed
-
- Lippard S. J. Science. 1986;233:992. - PubMed
-
- Solomon E. I. Baldwin M. J. Lowery M. D. Chem. Rev. 1992;92:521–542.
-
- Remenyi C. Reviakine R. Kaupp M. J. Phys. Chem. B. 2007;111:8290–8304. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

