Tolerability profile of paliperidone palmitate formulations: A pharmacovigilance analysis of the EUDRAVigilance database
- PMID: 37091708
- PMCID: PMC10116827
- DOI: 10.3389/fpsyt.2023.1130636
Tolerability profile of paliperidone palmitate formulations: A pharmacovigilance analysis of the EUDRAVigilance database
Abstract
Introduction: Long-acting injectable antipsychotics (LAIs) have proven to be effective in the maintenance treatment of patients suffering from schizophrenia, and their safety and tolerability profiles represent a key factor in their long-term use and choice in clinical practice. Paliperidone palmitate (PP) is the only second-generation LAI (SGA-LAI), available in both one- (PP1M) and 3-month (PP3M) formulations. However, real-world prospective studies on PP1M and PP3M are still few and mostly conducted on small samples. In this context, we aimed to better define the safety and tolerability profile of PP using real world pharmacovigilance data.
Methods: We retrospectively analyzed the publicly available data regarding Individual Case Safety Reports (ICSRs), presenting PP1M and/or PP3M as suspected drugs, reported on EUDRAVigilance between 2011 and June 30th, 2022. ICSRs relative to at least one SGA-LAI other than PP, reported between 2003 and June 30th, 2022, were also examined as reference group. Data were evaluated with a descriptive analysis, and then, as disproportionality measures, crude reporting odds ratio (ROR) and 95% confidence interval (CI) were calculated.
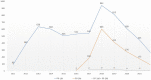
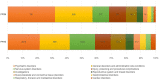
Results: A total of 8,152 ICSRs met the inclusion criteria, of those 77.7% (n = 6,332) presented as suspected drug PP1M, 21.2% (n = 1,731) PP3M, while 89 cases indicated both PP1M and PP3M. Significantly higher probabilities of reporting in PP-related reports were observed for the primary Standardized MedDRA Queries "Sexual Dysfunctions" (ROR = 1.45; 95% CI 1.23-1.70), "Haemodynamic oedema, effusions and fluid overload" (ROR = 1.42; 1.18-1.70), as well as "Fertility disorders" (ROR = 2.69; 1.51-4.80).
Discussion: Our analysis indicates that the tolerability and safety profiles of PP are in line with what is known for the other SGA-LAIs. However, differences regarding endocrine system ADRs have been noticed. The results presented in this work do not discourage the prescription of SGA-LAI formulations but aim to enhance their safety.
Keywords: EUDRAVigliance; adverse drug reaction; antipsychotics; drug-induced reaction; long-acting injectable; paliperidone palmitate; pharmacovigilance; schizophrenia.
Copyright © 2023 Cicala, de Filippis, Barbieri, Cutroneo, De Fazio, Schoretsanitis and Spina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Effectiveness of SGA-LAIs on Clinical, Cognitive, and Social Domains in Schizophrenia: Results from a Prospective Naturalistic Study.Brain Sci. 2023 Mar 29;13(4):577. doi: 10.3390/brainsci13040577. Brain Sci. 2023. PMID: 37190542 Free PMC article.
-
Cost-effectiveness analysis of monthly, 3-monthly, and 6-monthly long-acting injectable and oral paliperidone in adults with schizophrenia.J Manag Care Spec Pharm. 2023 Aug;29(8):884-895. doi: 10.18553/jmcp.2023.29.8.884. J Manag Care Spec Pharm. 2023. PMID: 37523313 Free PMC article.
-
Evaluating the 6-month formulation of paliperidone palmitate: a twice-yearly injectable treatment for schizophrenia in adults.Expert Rev Neurother. 2024 Apr;24(4):325-332. doi: 10.1080/14737175.2024.2325655. Epub 2024 Mar 6. Expert Rev Neurother. 2024. PMID: 38445396 Review.
-
Impact of 3-Monthly Long-Acting Injectable Paliperidone Palmitate in Schizophrenia: A Retrospective, Real-World Analysis of Population-Based Health Records in Spain.CNS Drugs. 2022 May;36(5):517-527. doi: 10.1007/s40263-022-00917-1. Epub 2022 Apr 23. CNS Drugs. 2022. PMID: 35460508 Free PMC article.
-
Role of paliperidone palmitate 3-monthly in the management of schizophrenia: insights from clinical practice.Neuropsychiatr Dis Treat. 2019 Feb 11;15:449-456. doi: 10.2147/NDT.S140383. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 30804673 Free PMC article. Review.
Cited by
-
Effectiveness of SGA-LAIs on Clinical, Cognitive, and Social Domains in Schizophrenia: Results from a Prospective Naturalistic Study.Brain Sci. 2023 Mar 29;13(4):577. doi: 10.3390/brainsci13040577. Brain Sci. 2023. PMID: 37190542 Free PMC article.
-
Safety of Inclisiran: A Disproportionality Analysis from the EudraVigilance Database.Pharmaceuticals (Basel). 2024 Oct 12;17(10):1365. doi: 10.3390/ph17101365. Pharmaceuticals (Basel). 2024. PMID: 39459005 Free PMC article.
-
Safety of Dual Orexin Receptor Antagonist Daridorexant: A Disproportionality Analysis of Publicly Available FAERS Data.Pharmaceuticals (Basel). 2024 Mar 6;17(3):342. doi: 10.3390/ph17030342. Pharmaceuticals (Basel). 2024. PMID: 38543128 Free PMC article.
-
Neuropsychiatric Adverse Events with Monoclonal Antibodies Approved for Multiple Myeloma: An Analysis from the FDA Adverse Event Reporting System.Pharmaceuticals (Basel). 2024 Sep 25;17(10):1266. doi: 10.3390/ph17101266. Pharmaceuticals (Basel). 2024. PMID: 39458907 Free PMC article.
References
-
- de Bartolomeis A, Barone A, Begni V, Riva MA. Present and future antipsychotic drugs: a systematic review of the putative mechanisms of action for efficacy and a critical appraisal under a translational perspective. Pharmacol Res. (2022) 176:106078. doi: 10.1016/j.phrs.2022.106078, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

