Multiple transcriptome comparisons reveal the essential roles of FLOWERING LOCUS T in floral initiation and SOC1 and SVP in floral activation in blueberry
- PMID: 37091803
- PMCID: PMC10113452
- DOI: 10.3389/fgene.2023.1105519
Multiple transcriptome comparisons reveal the essential roles of FLOWERING LOCUS T in floral initiation and SOC1 and SVP in floral activation in blueberry
Abstract
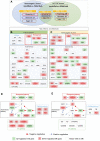
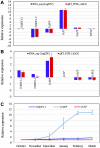
The flowering mechanisms, especially chilling requirement-regulated flowering, in deciduous woody crops remain to be elucidated. Flower buds of northern highbush blueberry cultivar Aurora require approximately 1,000 chilling hours to bloom. Overexpression of a blueberry FLOWERING LOCUS T (VcFT) enabled precocious flowering of transgenic "Aurora" mainly in non-terminated apical buds during flower bud formation, meanwhile, most of the mature flower buds could not break until they received enough chilling hours. In this study, we highlighted two groups of differentially expressed genes (DEGs) in flower buds caused by VcFT overexpression (VcFT-OX) and full chilling. We compared the two groups of DEGs with a focus on flowering pathway genes. We found: 1) In non-chilled flower buds, VcFT-OX drove a high VcFT expression and repressed expression of a major MADS-box gene, blueberry SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (VcSOC1) resulting an increased VcFT/VcSOC1 expression ratio; 2) In fully chilled flower buds that are ready to break, the chilling upregulated VcSOC1 expression in non-transgenic "Aurora" and repressed VcFT expression in VcFT-OX "Aurora", and each resulted in a decreased ratio of VcFT to VcSOC1; additionally, expression of a blueberry SHORT VEGETATIVE PHASE (VcSVP) was upregulated in chilled flower buds of both transgenic and non-transgenic' "Aurora". Together with additional analysis of VcFT and VcSOC1 in the transcriptome data of other genotypes and tissues, we provide evidence to support that VcFT expression plays a significant role in promoting floral initiation and that VcSOC1 expression is a key floral activator. We thus propose a new hypothesis on blueberry flowering mechanism, of which the ratios of VcFT-to-VcSOC1 at transcript levels in the flowering pathways determine flower bud formation and bud breaking. Generally, an increased VcFT/VcSOC1 ratio or increased VcSOC1 in leaf promotes precocious flowering and flower bud formation, and a decreased VcFT/VcSOC1 ratio with increased VcSOC1 in fully chilled flower buds contributes to flower bud breaking.
Keywords: FLOWERING LOCUS T; SHORT VEGETATIVE PHASE; SUPPRESSOR OF OVEREXPRESSION OF CONSTAN 1; Vaccinium corymbosum; chilling requirement; dormancy release; flowering mechanism; transcriptome analysis.
Copyright © 2023 Song, Carter and Zhong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Regulatory frameworks involved in the floral induction, formation and developmental programming of woody horticultural plants: a case study on blueberries.Front Plant Sci. 2024 Feb 12;15:1336892. doi: 10.3389/fpls.2024.1336892. eCollection 2024. Front Plant Sci. 2024. PMID: 38410737 Free PMC article. Review.
-
Comparative transcriptome analysis of nonchilled, chilled, and late-pink bud reveals flowering pathway genes involved in chilling-mediated flowering in blueberry.BMC Plant Biol. 2018 May 31;18(1):98. doi: 10.1186/s12870-018-1311-8. BMC Plant Biol. 2018. PMID: 29855262 Free PMC article.
-
The Vaccinium corymbosum FLOWERING LOCUS T-like gene (VcFT): a flowering activator reverses photoperiodic and chilling requirements in blueberry.Plant Cell Rep. 2013 Nov;32(11):1759-69. doi: 10.1007/s00299-013-1489-z. Epub 2013 Aug 2. Plant Cell Rep. 2013. PMID: 23907615
-
Overexpression of blueberry FLOWERING LOCUS T is associated with changes in the expression of phytohormone-related genes in blueberry plants.Hortic Res. 2016 Oct 26;3:16053. doi: 10.1038/hortres.2016.53. eCollection 2016. Hortic Res. 2016. PMID: 27818778 Free PMC article.
-
Beyond floral initiation: the role of flower bud dormancy in flowering time control of annual plants.J Exp Bot. 2024 Oct 16;75(19):6056-6062. doi: 10.1093/jxb/erae223. J Exp Bot. 2024. PMID: 38795335 Free PMC article. Review.
Cited by
-
Regulatory frameworks involved in the floral induction, formation and developmental programming of woody horticultural plants: a case study on blueberries.Front Plant Sci. 2024 Feb 12;15:1336892. doi: 10.3389/fpls.2024.1336892. eCollection 2024. Front Plant Sci. 2024. PMID: 38410737 Free PMC article. Review.
-
Gene and Its Promoter Cloning, and Functional Validation of JmSOC1 Revealed Its Role in Promoting Early Flowering and the Interaction with the JmSVP Protein.Int J Mol Sci. 2024 Dec 1;25(23):12932. doi: 10.3390/ijms252312932. Int J Mol Sci. 2024. PMID: 39684642 Free PMC article.
-
Modeling the Flowering Activation Motif during Vernalization in Legumes: A Case Study of M. trancatula.Life (Basel). 2023 Dec 23;14(1):26. doi: 10.3390/life14010026. Life (Basel). 2023. PMID: 38255642 Free PMC article.
-
Transcriptome-Based Identification of Candidate Flowering-Associated Genes of Blueberry in a Plant Factory with Artificial Lighting (PFAL) under Short-Day-Length Conditions.Int J Mol Sci. 2024 Mar 11;25(6):3197. doi: 10.3390/ijms25063197. Int J Mol Sci. 2024. PMID: 38542171 Free PMC article.
-
The Molecular Mechanism of Interaction Between SEPALLATA3 and APETALA1 in Arabidopsis thaliana.Plant Direct. 2025 Mar 30;9(4):e70052. doi: 10.1002/pld3.70052. eCollection 2025 Apr. Plant Direct. 2025. PMID: 40166359 Free PMC article.
References
-
- Atkinson C. J., Brennan R. M., Jones H. G. (2013). Declining chilling and its impact on temperate perennial crops. Environ. Exp. Bot. 91, 48–62. 10.1016/j.envexpbot.2013.02.004 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

