SARS-CoV-2 infection and immune responses
- PMID: 37091818
- PMCID: PMC10113164
- DOI: 10.3934/microbiol.2023015
SARS-CoV-2 infection and immune responses
Abstract
The recent pandemic caused by the SARS-CoV-2 virus continues to be an enormous global challenge faced by the healthcare sector. Availability of new vaccines and drugs targeting SARS-CoV-2 and sequelae of COVID-19 has given the world hope in ending the pandemic. However, the emergence of mutations in the SARS-CoV-2 viral genome every couple of months in different parts of world is a persistent danger to public health. Currently there is no single treatment to eradicate the risk of COVID-19. The widespread transmission of SARS-CoV-2 due to the Omicron variant necessitates continued work on the development and implementation of effective vaccines. Moreover, there is evidence that mutations in the receptor domain of the SARS-CoV-2 spike glycoprotein led to the decrease in current vaccine efficacy by escaping antibody recognition. Therefore, it is essential to actively identify the mechanisms by which SARS-CoV-2 evades the host immune system, study the long-lasting effects of COVID-19 and develop therapeutics targeting SARS-CoV-2 infections in humans and preclinical models. In this review, we describe the pathogenic mechanisms of SARS-CoV-2 infection as well as the innate and adaptive host immune responses to infection. We address the ongoing need to develop effective vaccines that provide protection against different variants of SARS-CoV-2, as well as validated endpoint assays to evaluate the immunogenicity of vaccines in the pipeline, medications, anti-viral drug therapies and public health measures, that will be required to successfully end the COVID-19 pandemic.
Keywords: COVID-19; SARS-CoV-2; antibodies; immunopathology; inflammation; vaccines.
© 2023 the Author(s), licensee AIMS Press.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures
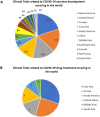
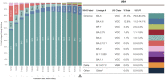


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
