An update on lateral flow immunoassay for the rapid detection of SARS-CoV-2 antibodies
- PMID: 37091823
- PMCID: PMC10113162
- DOI: 10.3934/microbiol.2023020
An update on lateral flow immunoassay for the rapid detection of SARS-CoV-2 antibodies
Abstract
Over the last three years, after the outbreak of the COVID-19 pandemic, an unprecedented number of novel diagnostic tests have been developed. Assays to evaluate the immune response to SARS-CoV-2 have been widely considered as part of the control strategy. The lateral flow immunoassay (LFIA), to detect both IgM and IgG against SARS-CoV-2, has been widely studied as a point-of-care (POC) test. Compared to laboratory tests, LFIAs are faster, cheaper and user-friendly, thus available also in areas with low economic resources. Soon after the onset of the pandemic, numerous kits for rapid antibody detection were put on the market with an emergency use authorization. However, since then, scientists have tried to better define the accuracy of these tests and their usefulness in different contexts. In fact, while during the first phase of the pandemic LFIAs for antibody detection were auxiliary to molecular tests for the diagnosis of COVID-19, successively these tests became a tool of seroprevalence surveillance to address infection control policies. When in 2021 a massive vaccination campaign was implemented worldwide, the interest in LFIA reemerged due to the need to establish the extent and the longevity of immunization in the vaccinated population and to establish priorities to guide health policies in low-income countries with limited access to vaccines. Here, we summarize the accuracy, the advantages and limits of LFIAs as POC tests for antibody detection, highlighting the efforts that have been made to improve this technology over the last few years.
Keywords: COVID-19; IgG; IgM; SARS-CoV-2; lateral flow immunoassay; neutralizing antibodies; point of care test; rapid antibody test.
© 2023 the Author(s), licensee AIMS Press.
Conflict of interest statement
Conflict of interest: All authors declare no conflicts of interest in this paper.
Figures
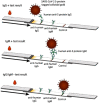
References
-
- Mathieu E, Ritchie H, Rodés-Guirao L, et al. Coronavirus Pandemic (COVID-19) 2020. Available from: https://ourworldindata.org/coronavirus.
-
- World Health Organization. Diagnostic testing for SARS-CoV-2. 2020. Available from: https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2.
-
- European Centre for Disease Prevention and Control. Considerations for the use of antibody tests for SARSCoV-2 – first update. Stockholm: ECDC; 2022.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
