Missed meal boluses and poorer glycemic control impact on neurocognitive function may be associated with white matter integrity in adolescents with type 1 diabetes
- PMID: 37091855
- PMCID: PMC10113499
- DOI: 10.3389/fendo.2023.1141085
Missed meal boluses and poorer glycemic control impact on neurocognitive function may be associated with white matter integrity in adolescents with type 1 diabetes
Abstract
Background: The notion that pediatric type 1 diabetes impacts brain function and structure early in life is of great concern. Neurological manifestations, including neurocognitive and behavioral symptoms, may be present from childhood, initially mild and undetectable in daily life. Despite intensive management and technological therapeutic interventions, most pediatric patients do not achieve glycemic control targets for HbA1c. One of the most common causes of such poor control and frequent transient hyperglycemic episodes may be lifestyle factors, including missed meal boluses.
Objective: The aim of this study was to assess the association between specific neurocognitive accomplishments-learning and memory, inhibition ability learning, and verbal and semantic memory-during meals with and without bolusing, correlated to diffusion tensor imaging measurements of major related tracts, and glycemic control in adolescents with type 1 diabetes compared with their healthy siblings of similar age.
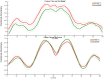
Study design and methods: This is a case-control study of 12- to 18-year-old patients with type 1 diabetes (N = 17, 8 male patients, diabetes duration of 6.53 ± 4.1 years) and their healthy siblings (N = 13). All were hospitalized for 30 h for continuous glucose monitoring and repeated neurocognitive tests as a function of a missed or appropriate pre-meal bolus. This situation was mimicked by controlled, patient blinded manipulation of lunch pre-meal bolus administration to enable capillary glucose level of <180 mg/dl and to >240 mg/d 2 hours after similar meals, at a similar time. The diabetes team randomly and blindly manipulated post-lunch glucose levels by subcutaneous injection of either rapid-acting insulin or 0.9% NaCl solution before lunch. A specific neurocognitive test battery was performed twice, after each manipulation, and its results were compared, along with additional neurocognitive tasks administered during hospitalization without insulin manipulation. Participants underwent brain imaging, including diffusion tensor imaging and tractography.
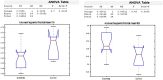
Results: A significant association was demonstrated between glycemic control and performance in the domains of executive functions, inhibition ability, learning and verbal memory, and semantic memory. Inhibition ability was specifically related to food management. Poorer glycemic control (>8.3%) was associated with a slower reaction time.
Conclusion: These findings highlight the potential impairment of brain networks responsible for learning, memory, and controlled reactivity to food in adolescents with type 1 diabetes whose glycemic control is poor.
Keywords: adolescents; brain domains; cognitive performance; diffusion tension imaging; glycemic control; type 1 diabetes.
Copyright © 2023 Litmanovitch, Geva, Leshem, Lezinger, Heyman, Gidron, Yarmolovsky, Sasson, Tal and Rachmiel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Mealtime insulin bolus adherence and glycemic control in adolescents on insulin pump therapy.Eur J Pediatr. 2018 Dec;177(12):1831-1836. doi: 10.1007/s00431-018-3256-1. Epub 2018 Sep 20. Eur J Pediatr. 2018. PMID: 30238153
-
The effectiveness of continuous subcutaneous insulin pumps with continuous glucose monitoring in outpatient adolescents with type 1 diabetes: A systematic review.JBI Libr Syst Rev. 2012;10(42 Suppl):1-10. doi: 10.11124/jbisrir-2012-170. JBI Libr Syst Rev. 2012. PMID: 27820140
-
Comparison between preprandial vs. postprandial insulin aspart in patients with type 1 diabetes on insulin pump and real-time continuous glucose monitoring.Diabetes Metab Res Rev. 2018 Sep;34(6):e3019. doi: 10.1002/dmrr.3019. Epub 2018 Jun 1. Diabetes Metab Res Rev. 2018. PMID: 29749032 Clinical Trial.
-
Insulin therapy in children and adolescents with type 1 diabetes.Paediatr Drugs. 2014 Apr;16(2):141-50. doi: 10.1007/s40272-014-0064-6. Paediatr Drugs. 2014. PMID: 24458650 Review.
-
Nutrition and Glycemic Control in Children and Adolescents with Type 1 Diabetes Mellitus Attending Diabetes Camps.Nutrients. 2024 Oct 1;16(19):3338. doi: 10.3390/nu16193338. Nutrients. 2024. PMID: 39408305 Free PMC article. Review.
References
-
- Miles R, Root F. Psychologic tests applied to diabetic patients. Arch Intern Med (1922) 30:767–77. doi: 10.1001/archinte.1922.00110120086003 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

