V-ATPase modulates exocytosis in neuroendocrine cells through the activation of the ARNO-Arf6-PLD pathway and the synthesis of phosphatidic acid
- PMID: 37091866
- PMCID: PMC10119424
- DOI: 10.3389/fmolb.2023.1163545
V-ATPase modulates exocytosis in neuroendocrine cells through the activation of the ARNO-Arf6-PLD pathway and the synthesis of phosphatidic acid
Abstract
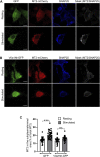
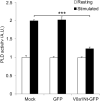
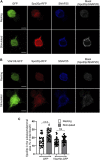
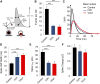
Although there is mounting evidence indicating that lipids serve crucial functions in cells and are implicated in a growing number of human diseases, their precise roles remain largely unknown. This is particularly true in the case of neurosecretion, where fusion with the plasma membrane of specific membrane organelles is essential. Yet, little attention has been given to the role of lipids. Recent groundbreaking research has emphasized the critical role of lipid localization at exocytotic sites and validated the essentiality of fusogenic lipids, such as phospholipase D (PLD)-generated phosphatidic acid (PA), during membrane fusion. Nevertheless, the regulatory mechanisms synchronizing the synthesis of these key lipids and neurosecretion remain poorly understood. The vacuolar ATPase (V-ATPase) has been involved both in vesicle neurotransmitter loading and in vesicle fusion. Thus, it represents an ideal candidate to regulate the fusogenic status of secretory vesicles according to their replenishment state. Indeed, the cytosolic V1 and vesicular membrane-associated V0 subdomains of V-ATPase were shown to dissociate during the stimulation of neurosecretory cells. This allows the subunits of the vesicular V0 to interact with different proteins of the secretory machinery. Here, we show that V0a1 interacts with the Arf nucleotide-binding site opener (ARNO) and promotes the activation of the Arf6 GTPase during the exocytosis in neuroendocrine cells. When the interaction between V0a1 and ARNO was disrupted, it resulted in the inhibition of PLD activation, synthesis of phosphatidic acid during exocytosis, and changes in the timing of fusion events. These findings indicate that the separation of V1 from V0 could function as a signal to initiate the ARNO-Arf6-PLD1 pathway and facilitate the production of phosphatidic acid, which is essential for effective exocytosis in neuroendocrine cells.
Keywords: V-ATPase; chromaffin cells; membrane fusion; neurosecretion; phospholipase D; phospholipids.
Copyright © 2023 Wang, Wolf, Ozkan, Richert, Mely, Chasserot-Golaz, Ory, Gasman and Vitale.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Calcium-regulated exocytosis of dense-core vesicles requires the activation of ADP-ribosylation factor (ARF)6 by ARF nucleotide binding site opener at the plasma membrane.J Cell Biol. 2002 Oct 14;159(1):79-89. doi: 10.1083/jcb.200203027. Epub 2002 Oct 14. J Cell Biol. 2002. PMID: 12379803 Free PMC article.
-
Regulated secretion in chromaffin cells: an essential role for ARF6-regulated phospholipase D in the late stages of exocytosis.Ann N Y Acad Sci. 2002 Oct;971:193-200. doi: 10.1111/j.1749-6632.2002.tb04463.x. Ann N Y Acad Sci. 2002. PMID: 12438119
-
Identification of a plasma membrane-associated guanine nucleotide exchange factor for ARF6 in chromaffin cells. Possible role in the regulated exocytotic pathway.J Biol Chem. 2000 May 26;275(21):15637-44. doi: 10.1074/jbc.M908347199. J Biol Chem. 2000. PMID: 10748097
-
Synthesis of fusogenic lipids through activation of phospholipase D1 by GTPases and the kinase RSK2 is required for calcium-regulated exocytosis in neuroendocrine cells.Biochem Soc Trans. 2010 Feb;38(Pt 1):167-71. doi: 10.1042/BST0380167. Biochem Soc Trans. 2010. PMID: 20074053 Review.
-
Signalling role for ARF and phospholipase D in mast cell exocytosis stimulated by crosslinking of the high affinity FcepsilonR1 receptor.Mol Immunol. 2002 Sep;38(16-18):1277-82. doi: 10.1016/s0161-5890(02)00075-5. Mol Immunol. 2002. PMID: 12217395 Review.
Cited by
-
The dynamic regulatory network of phosphatidic acid metabolism: a spotlight on substrate cycling between phosphatidic acid and diacylglycerol.Biochem Soc Trans. 2024 Oct 30;52(5):2123-2132. doi: 10.1042/BST20231511. Biochem Soc Trans. 2024. PMID: 39417337 Free PMC article. Review.
-
The spectrum of lysosomal stress and damage responses: from mechanosensing to inflammation.EMBO Rep. 2025 Mar;26(6):1425-1439. doi: 10.1038/s44319-025-00405-9. Epub 2025 Feb 27. EMBO Rep. 2025. PMID: 40016424 Free PMC article. Review.
-
Designing New Natural-Mimetic Phosphatidic Acid: A Versatile and Innovative Synthetic Strategy for Glycerophospholipid Research.Angew Chem Int Ed Engl. 2025 Sep 1;64(36):e202510412. doi: 10.1002/anie.202510412. Epub 2025 Jul 25. Angew Chem Int Ed Engl. 2025. PMID: 40626950 Free PMC article.
-
Phospholipase D1 produces phosphatidic acid at sites of secretory vesicle docking and fusion.Mol Biol Cell. 2024 Mar 1;35(3):ar39. doi: 10.1091/mbc.E23-05-0189. Epub 2023 Dec 20. Mol Biol Cell. 2024. PMID: 38117597 Free PMC article.
References
-
- Caumont A. S., Vitale N., Gensse M., Galas M. C., Casanova J. E., Bader M. F. (2000). Identification of a plasma membrane-associated guanine nucleotide exchange factor for ARF6 in chromaffin cells. Possible role in the regulated exocytotic pathway. J. Biol. Chem. 275 (21), 15637–15644. 10.1074/jbc.M908347199 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

