Early life adversity impaired dorsal striatal synaptic transmission and behavioral adaptability to appropriate action selection in a sex-dependent manner
- PMID: 37091877
- PMCID: PMC10116150
- DOI: 10.3389/fnsyn.2023.1128640
Early life adversity impaired dorsal striatal synaptic transmission and behavioral adaptability to appropriate action selection in a sex-dependent manner
Abstract
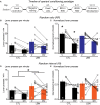
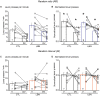
Early life adversity (ELA) is a major health burden in the United States, with 62% of adults reporting at least one adverse childhood experience. These experiences during critical stages of brain development can perturb the development of neural circuits that mediate sensory cue processing and behavioral regulation. Recent studies have reported that ELA impaired the maturation of dendritic spines on neurons in the dorsolateral striatum (DLS) but not in the dorsomedial striatum (DMS). The DMS and DLS are part of two distinct corticostriatal circuits that have been extensively implicated in behavioral flexibility by regulating and integrating action selection with the reward value of those actions. To date, no studies have investigated the multifaceted effects of ELA on aspects of behavioral flexibility that require alternating between different action selection strategies or higher-order cognitive processes, and the underlying synaptic transmission in corticostriatal circuitries. To address this, we employed whole-cell patch-clamp electrophysiology to assess the effects of ELA on synaptic transmission in the DMS and DLS. We also investigated the effects of ELA on the ability to update action control in response to outcome devaluation in an instrumental learning paradigm and reversal of action-outcome contingency in a water T-maze paradigm. At the circuit level, ELA decreased corticostriatal glutamate transmission in male but not in female mice. Interestingly, in DMS, glutamate transmission is decreased in male ELA mice, but increased in female ELA mice. ELA impaired the ability to update action control in response to reward devaluation in a context that promotes goal-directedness in male mice and induced deficits in reversal learning. Overall, our findings demonstrate the sex- and region-dependent effects of ELA on behavioral flexibility and underlying corticostriatal glutamate transmission. By establishing a link between ELA and circuit mechanisms underlying behavioral flexibility, our findings will begin to identify novel molecular mechanisms that can represent strategies for treating behavioral inflexibility in individuals who experienced early life traumatic incidents.
Keywords: action-outcome contingency; behavioral flexibility; corticostriatal circuits; early life adversity; outcome devaluation; reversal learning; synaptic transmission.
Copyright © 2023 de Carvalho, Khoja, Haile and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Early-life adversity selectively interrupts the dendritic differentiation of dorsolateral striatal neurons in male mice.Brain Struct Funct. 2021 Mar;226(2):397-414. doi: 10.1007/s00429-020-02183-7. Epub 2021 Jan 2. Brain Struct Funct. 2021. PMID: 33386419
-
The Impacts of Early-life Adversity on Striatal and Hippocampal Memory Functions.Neuroscience. 2022 May 10;490:11-24. doi: 10.1016/j.neuroscience.2022.02.029. Epub 2022 Mar 4. Neuroscience. 2022. PMID: 35248584
-
Region-specific impairments in striatal synaptic transmission and impaired instrumental learning in a mouse model of Angelman syndrome.Eur J Neurosci. 2014 Mar;39(6):1018-1025. doi: 10.1111/ejn.12442. Epub 2013 Dec 13. Eur J Neurosci. 2014. PMID: 24329862 Free PMC article.
-
Neurodevelopmental origins of substance use disorders: Evidence from animal models of early-life adversity and addiction.Eur J Neurosci. 2022 May;55(9-10):2170-2195. doi: 10.1111/ejn.15223. Epub 2021 Apr 25. Eur J Neurosci. 2022. PMID: 33825217 Free PMC article. Review.
-
Neural circuit mechanism for learning dependent on dopamine transmission: roles of striatal direct and indirect pathways in sensory discrimination.Adv Pharmacol. 2013;68:143-53. doi: 10.1016/B978-0-12-411512-5.00007-5. Adv Pharmacol. 2013. PMID: 24054143 Review.
Cited by
-
Probing the genomic landscape of human sexuality: a critical systematic review of the literature.Front Genet. 2023 Aug 24;14:1184758. doi: 10.3389/fgene.2023.1184758. eCollection 2023. Front Genet. 2023. PMID: 37693319 Free PMC article.
-
Reconfiguration of brain-wide neural activity after early life adversity.bioRxiv [Preprint]. 2025 May 29:2023.09.10.557058. doi: 10.1101/2023.09.10.557058. bioRxiv. 2025. PMID: 38328213 Free PMC article. Preprint.
-
Conditional deletion of neurexin-2 impaired behavioral flexibility to alterations in action-outcome contingency.Sci Rep. 2024 May 3;14(1):10187. doi: 10.1038/s41598-024-60760-w. Sci Rep. 2024. PMID: 38702381 Free PMC article.
References
-
- Arp J. M., Ter Horst J. P., Loi M., den Blaauwen J., Bangert E., Fernández G., et al. . (2016). Blocking glucocorticoid receptors at adolescent age prevents enhanced freezing between repeated cue-exposures after conditioned fear in adult mice raised under chronic early life stress. Neurobiol. Learn. Mem. 133, 30–38. 10.1016/j.nlm.2016.05.009 - DOI - PubMed

