Cryo-EM tomography and automatic segmentation delineate modular structures in the postsynaptic density
- PMID: 37091879
- PMCID: PMC10117989
- DOI: 10.3389/fnsyn.2023.1123564
Cryo-EM tomography and automatic segmentation delineate modular structures in the postsynaptic density
Abstract
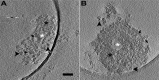
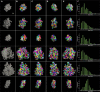
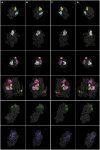
Postsynaptic densities (PSDs) are large protein complexes associated with the postsynaptic membrane of excitatory synapses important for synaptic function including plasticity. Conventional electron microscopy (EM) typically depicts PSDs as compact disk-like structures of hundreds of nanometers in size. Biochemically isolated PSDs were also similar in dimension revealing a predominance of proteins with the ability to polymerize into an extensive scaffold; several EM studies noted their irregular contours with often small granular structures (<30 nm) and holes. Super-resolution light microscopy studies observed clusters of PSD elements and their activity-induced lateral movement. Furthermore, our recent EM study on PSD fractions after sonication observed PSD fragments (40-90 nm in size) separate from intact PSDs; however, such structures within PSDs remained unidentified. Here we examined isolated PSDs by cryo-EM tomography with our new approach of automatic segmentation that enables delineation of substructures and their quantitative analysis. The delineated substructures broadly varied in size, falling behind 30 nm or exceeding 100 nm and showed that a considerable portion of the substructures (>38%) in isolated PSDs was in the same size range as those fragments. Furthermore, substructures spanning the entire thickness of the PSD were found, large enough to contain both membrane-associated and cytoplasmic proteins of the PSD; interestingly, they were similar to nanodomains in frequency. The structures detected here appear to constitute the isolated PSD as modules of various compositions, and this modular nature may facilitate remodeling of the PSD for proper synaptic function and plasticity.
Keywords: automatic segmentation; cryo-EM tomography; modular organization; nanodomain; postsynaptic density.
Copyright © 2023 Jung, Chen and Reese.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Differentiation and Characterization of Excitatory and Inhibitory Synapses by Cryo-electron Tomography and Correlative Microscopy.J Neurosci. 2018 Feb 7;38(6):1493-1510. doi: 10.1523/JNEUROSCI.1548-17.2017. Epub 2018 Jan 8. J Neurosci. 2018. PMID: 29311144 Free PMC article.
-
Postsynaptic densities fragment into subcomplexes upon sonication.Mol Brain. 2019 Aug 22;12(1):72. doi: 10.1186/s13041-019-0491-y. Mol Brain. 2019. PMID: 31439005 Free PMC article.
-
Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD.J Neurosci. 2003 Dec 3;23(35):11270-8. doi: 10.1523/JNEUROSCI.23-35-11270.2003. J Neurosci. 2003. PMID: 14657186 Free PMC article.
-
[Molecular mechanism underlying the formation and maintenance of excitatory synapses].Brain Nerve. 2011 Jan;63(1):51-8. Brain Nerve. 2011. PMID: 21228448 Review. Japanese.
-
The postsynaptic architecture of excitatory synapses: a more quantitative view.Annu Rev Biochem. 2007;76:823-47. doi: 10.1146/annurev.biochem.76.060805.160029. Annu Rev Biochem. 2007. PMID: 17243894 Review.
Cited by
-
Tubulin and GTP Are Crucial Elements for Postsynaptic Density Construction and Aggregation.J Neurochem. 2025 May;169(5):e70085. doi: 10.1111/jnc.70085. J Neurochem. 2025. PMID: 40396438 Free PMC article.
-
The postsynaptic density in excitatory synapses is composed of clustered, heterogeneous nanoblocks.J Cell Biol. 2025 Jun 2;224(6):e202406133. doi: 10.1083/jcb.202406133. Epub 2025 Mar 27. J Cell Biol. 2025. PMID: 40145863 Free PMC article.
-
Activity-dependent diffusion trapping of AMPA receptors as a key step for expression of early LTP.Philos Trans R Soc Lond B Biol Sci. 2024 Jul 29;379(1906):20230220. doi: 10.1098/rstb.2023.0220. Epub 2024 Jun 10. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38853553 Free PMC article. Review.
-
Sonication dissociates the synaptic cleft and allows purification of postsynaptic densities with associated postsynaptic membrane.Mol Brain. 2025 May 30;18(1):47. doi: 10.1186/s13041-025-01217-7. Mol Brain. 2025. PMID: 40448157 Free PMC article.
References
LinkOut - more resources
Full Text Sources

