Microbial Biotransformation Products and Pathways of Dichloroacetamide Herbicide Safeners
- PMID: 37091899
- PMCID: PMC10111411
- DOI: 10.1021/acs.estlett.2c00862
Microbial Biotransformation Products and Pathways of Dichloroacetamide Herbicide Safeners
Abstract
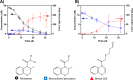
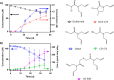
Dichloroacetamide safeners are common ingredients in commercial herbicide formulations. We previously investigated the environmental fate of dichloroacetamides via photolysis and hydrolysis, but other potentially important, environmentally relevant fate processes remain uncharacterized and may yield products of concern. Here, we examined microbial biotransformation of two dichloroacetamide safeners, benoxacor and dichlormid, to identify products and elucidate pathways. Using aerobic microcosms inoculated with river sediment, we demonstrated that microbial biotransformations of benoxacor and dichlormid proceed primarily, if not exclusively, via cometabolism. Benoxacor was transformed by both hydrolysis and microbial biotransformation processes; in most cases, biotransformation rates were faster than hydrolysis rates. We identified multiple novel products of benoxacor and dichlormid not previously observed for microbial processes, with several products similar to those reported for structurally related chloroacetamide herbicides, thus indicating potential for conserved biotransformation mechanisms across both chemical classes. Observed products include monochlorinated species such as the banned herbicide CDAA (from dichlormid), glutathione conjugates, and sulfur-containing species. We propose a transformation pathway wherein benoxacor and dichlormid are first dechlorinated, likely via microbial hydrolysis, and subsequently conjugated with glutathione. This is the first study reporting biological dechlorination of dichloroacetamides to yield monochlorinated products in aerobic environments.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Acid- and Base-Mediated Hydrolysis of Dichloroacetamide Herbicide Safeners.Environ Sci Technol. 2022 Jan 4;56(1):325-334. doi: 10.1021/acs.est.1c05958. Epub 2021 Dec 17. Environ Sci Technol. 2022. PMID: 34920670 Free PMC article.
-
Photochemical Transformations of Dichloroacetamide Safeners.Environ Sci Technol. 2019 Jun 18;53(12):6738-6746. doi: 10.1021/acs.est.9b00861. Epub 2019 May 31. Environ Sci Technol. 2019. PMID: 31117539
-
Black carbon-enhanced transformation of dichloroacetamide safeners: Role of reduced sulfur species.Sci Total Environ. 2020 Oct 10;738:139908. doi: 10.1016/j.scitotenv.2020.139908. Epub 2020 Jun 2. Sci Total Environ. 2020. PMID: 32531604
-
Herbicide safeners: uses, limitations, metabolism, and mechanisms of action.Chemosphere. 2002 Sep;48(9):965-74. doi: 10.1016/s0045-6535(02)00185-6. Chemosphere. 2002. PMID: 12222792 Review.
-
Review on the Discovery of Novel Natural Herbicide Safeners.J Agric Food Chem. 2023 Aug 2;71(30):11320-11331. doi: 10.1021/acs.jafc.3c03585. Epub 2023 Jul 19. J Agric Food Chem. 2023. PMID: 37466454 Review.
Cited by
-
A systematic review of herbicide safener toxicity.Crit Rev Toxicol. 2024 Nov;54(10):805-855. doi: 10.1080/10408444.2024.2391431. Epub 2024 Oct 1. Crit Rev Toxicol. 2024. PMID: 39351770
References
-
- Rosinger C. Herbicide Safeners: An Overview. Julius-Kuhn-Archiv 2014, 443, 516–525. 10.5073/jka.2014.443.066. - DOI
-
- Sivey J. D.; Lehmler H.-J.; Salice C. J.; Ricko A. N.; Cwiertny D. M. Environmental Fate and Effects of Dichloroacetamide Herbicide Safeners: “Inert” yet Biologically Active Agrochemical Ingredients. Environ. Sci. Technol. Lett. 2015, 2 (10), 260–269. 10.1021/acs.estlett.5b00220. - DOI
-
- Estimation Programs Interface Suite for Microsoft Windows, v 4.11; United States Environmental Protection Agency: Washington, DC,2014.
Grants and funding
LinkOut - more resources
Full Text Sources
