A Pilot Single-Blinded, Randomized, Controlled Trial Comparing BNT162b2 vs. JNJ-78436735 Vaccine as the Third Dose After Two Doses of BNT162b2 Vaccine in Solid Organ Transplant Recipients
- PMID: 37091963
- PMCID: PMC10113439
- DOI: 10.3389/ti.2023.10938
A Pilot Single-Blinded, Randomized, Controlled Trial Comparing BNT162b2 vs. JNJ-78436735 Vaccine as the Third Dose After Two Doses of BNT162b2 Vaccine in Solid Organ Transplant Recipients
Abstract
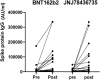
Solid Organ Transplant (SOT) recipients are at significant higher risk for COVID-19 and due to immunosuppressive medication, the immunogenicity after vaccination is suboptimal. In the previous studies, booster method showed significant benefit in this population. In the current study, we compared using a mix-and-match method vs. same vaccine as a third dose in SOT recipients. This was a patient-blinded, single center, randomized controlled trial comparing BNT162b2 vs. JNJ-78436735 vaccine as the third dose after two doses of BNT162b2 vaccine. We included adult SOT recipients with functional graft who had received two doses of BNT162b2 vaccine. Participants were randomly assigned to receive either BNT162b2 or JNJ-78436735 in one-to-one ratio. Primary outcome was SARS-CoV-2 IgG positivity at 1 month after the third dose. Sixty SOT recipients, including 36 kidney, 12 liver, 2 lung, 3 heart, and 5 combined transplants, were enrolled, and 57 recipients were analyzed per protocol. There were no statistically significant differences between the two vaccine protocols for IgG positivity (83.3% vs. 85.2% for BNT162b2 and JNJ-78436735, respectively, p = 0.85, Odds Ratio 0.95, 95% Confidence Interval 0.23-4.00). Comparison of the geometric mean titer demonstrated a higher trend with BNT162b2 (p = 0.09). In this pilot randomized controlled trial comparing mix and match method vs. uniform vaccination in SOT recipients, both vaccines were safely used. Since this was a small sample sized study, there was no statistically significant difference in immunogenicity; though, the mix and match method showed relatively lower geometric mean titer, as compared to uniform vaccine. Further studies need to be conducted to determine duration of this immunogenicity. Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT05047640?term=20210641&draw=2&rank=1, identifier 20210641.
Keywords: COVID-19; booster; randomized controlled trial; solid organ transplant; vaccine.
Copyright © 2023 Natori, Martin, Mattiazzi, Arosemena, Ortigosa-Goggins, Shobana, Roth, Kupin, Burke, Ciancio, Morsi, Phancao, Munagala, Butrous, Manickavel, Sinha, Sota, Pallikkuth, Bini, Simkins, Anjan, Vianna and Guerra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Post hoc analysis: 6 Months immunogenicity after third dose of BNT162b2 vs JNJ-78,436,735 after two doses of BNT162b2 vaccine in solid organ transplant recipients.Immunol Lett. 2025 Jun;273:106968. doi: 10.1016/j.imlet.2024.106968. Epub 2025 Jan 9. Immunol Lett. 2025. PMID: 39798807 Clinical Trial.
-
Decline in Antibody Concentration 6 Months After Two Doses of SARS-CoV-2 BNT162b2 Vaccine in Solid Organ Transplant Recipients and Healthy Controls.Front Immunol. 2022 Feb 23;13:832501. doi: 10.3389/fimmu.2022.832501. eCollection 2022. Front Immunol. 2022. PMID: 35281023 Free PMC article.
-
The Impact of Time between Booster Doses on Humoral Immune Response in Solid Organ Transplant Recipients Vaccinated with BNT162b2 Vaccines.Viruses. 2024 May 28;16(6):860. doi: 10.3390/v16060860. Viruses. 2024. PMID: 38932153 Free PMC article.
-
A Systematic Review and Meta-Analysis of Serologic Response following Coronavirus Disease 2019 (COVID-19) Vaccination in Solid Organ Transplant Recipients.Viruses. 2022 Aug 19;14(8):1822. doi: 10.3390/v14081822. Viruses. 2022. PMID: 36016444 Free PMC article.
-
Seroconversion rate after primary vaccination with two doses of BNT162b2 versus mRNA-1273 in solid organ transplant recipients: a systematic review and meta-analysis.Nephrol Dial Transplant. 2022 Jul 26;37(8):1566-1575. doi: 10.1093/ndt/gfac174. Nephrol Dial Transplant. 2022. PMID: 35544087 Free PMC article.
Cited by
-
Heterologous versus homologous COVID-19 booster vaccinations for adults: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials.BMC Med. 2024 Jun 24;22(1):263. doi: 10.1186/s12916-024-03471-3. BMC Med. 2024. PMID: 38915011 Free PMC article.
-
SARS-CoV-2 vaccine-elicited immune responses in solid organ transplant recipients.Clin Transplant Res. 2024 Dec 31;38(4):247-256. doi: 10.4285/ctr.24.0062. Clin Transplant Res. 2024. PMID: 39743229 Free PMC article. Review.
References
-
- O'Shaughnessy JA. Evusheld (Tixagevimab Co-packaged with Cilgavimab) - EUA Letter of Authorization [Letter of Authorization] (2022). Available from: https://www.fda.gov/media/154704/download (Accessed February 15, 2023).
-
- Manothummetha K, Chuleerarux N, Sanguankeo A, Kates OS, Hirankarn N, Thongkam A, et al. Immunogenicity and Risk Factors Associated with Poor Humoral Immune Response of SARS-CoV-2 Vaccines in Recipients of Solid Organ Transplant: A Systematic Review and Meta-Analysis. JAMA Netw Open (2022) 5(4):e226822. 10.1001/jamanetworkopen.2022.6822 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous