Modulation of T-cell function by myeloid-derived suppressor cells in hematological malignancies
- PMID: 37091970
- PMCID: PMC10113446
- DOI: 10.3389/fcell.2023.1129343
Modulation of T-cell function by myeloid-derived suppressor cells in hematological malignancies
Abstract
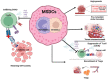
Myeloid-derived suppressor cells (MDSCs) are pathologically activated neutrophils and monocytes that negatively regulate the immune response to cancer and chronic infections. Abnormal myelopoiesis and pathological activation of myeloid cells generate this heterogeneous population of myeloid-derived suppressor cells. They are characterized by their distinct transcription, phenotypic, biochemical, and functional features. In the tumor microenvironment (TME), myeloid-derived suppressor cells represent an important class of immunosuppressive cells that correlate with tumor burden, stage, and a poor prognosis. Myeloid-derived suppressor cells exert a strong immunosuppressive effect on T-cells (and a broad range of other immune cells), by blocking lymphocyte homing, increasing production of reactive oxygen and nitrogen species, promoting secretion of various cytokines, chemokines, and immune regulatory molecules, stimulation of other immunosuppressive cells, depletion of various metabolites, and upregulation of immune checkpoint molecules. Additionally, the heterogeneity of myeloid-derived suppressor cells in cancer makes their identification challenging. Overall, they serve as a major obstacle for many cancer immunotherapies and targeting them could be a favorable strategy to improve the effectiveness of immunotherapeutic interventions. However, in hematological malignancies, particularly B-cell malignancies, the clinical outcomes of targeting these myeloid-derived suppressor cells is a field that is still to be explored. This review summarizes the complex biology of myeloid-derived suppressor cells with an emphasis on the immunosuppressive pathways used by myeloid-derived suppressor cells to modulate T-cell function in hematological malignancies. In addition, we describe the challenges, therapeutic strategies, and clinical relevance of targeting myeloid-derived suppressor cells in these diseases.
Keywords: hematological malignancies; immunosuppression; myeloid-derived suppressor cells; t cells; tumor microenvironment.
Copyright © 2023 Bhardwaj and Ansell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Myeloid-derived suppressor cells: key immunosuppressive regulators and therapeutic targets in hematological malignancies.Biomark Res. 2023 Mar 29;11(1):34. doi: 10.1186/s40364-023-00475-8. Biomark Res. 2023. PMID: 36978204 Free PMC article. Review.
-
The New Era of Cancer Immunotherapy: Targeting Myeloid-Derived Suppressor Cells to Overcome Immune Evasion.Front Immunol. 2020 Jul 30;11:1680. doi: 10.3389/fimmu.2020.01680. eCollection 2020. Front Immunol. 2020. PMID: 32849585 Free PMC article. Review.
-
Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression.Br J Cancer. 2019 Jan;120(1):16-25. doi: 10.1038/s41416-018-0333-1. Epub 2018 Nov 9. Br J Cancer. 2019. PMID: 30413826 Free PMC article. Review.
-
Myeloid-Derived Suppressor Cells in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1224:117-140. doi: 10.1007/978-3-030-35723-8_8. Adv Exp Med Biol. 2020. PMID: 32036608 Review.
-
Myeloid-derived suppressor cells in B cell malignancies.Tumour Biol. 2015 Sep;36(10):7339-53. doi: 10.1007/s13277-015-4004-z. Epub 2015 Sep 2. Tumour Biol. 2015. PMID: 26330296 Review.
Cited by
-
ALKBH5 regulates arginase 1 expression in MDSCs and their immunosuppressive activity in tumor-bearing host.Noncoding RNA Res. 2024 Mar 13;9(3):913-920. doi: 10.1016/j.ncrna.2024.03.003. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38638146 Free PMC article.
-
Therapeutic anti-cancer vaccines: a systematic review of prospective intervention trials for common hematological malignancies.EClinicalMedicine. 2025 Jul 22;86:103378. doi: 10.1016/j.eclinm.2025.103378. eCollection 2025 Aug. EClinicalMedicine. 2025. PMID: 40735344 Free PMC article. Review.
-
Promising Cellular Immunotherapy for Colorectal Cancer Using Classical Dendritic Cells and Natural Killer T Cells.Cells. 2025 Jan 22;14(3):166. doi: 10.3390/cells14030166. Cells. 2025. PMID: 39936958 Free PMC article. Review.
-
Unraveling the role of cancer-associated fibroblasts in B cell lymphoma.Front Immunol. 2024 Nov 1;15:1451791. doi: 10.3389/fimmu.2024.1451791. eCollection 2024. Front Immunol. 2024. PMID: 39555055 Free PMC article. Review.
-
Resveratrol Attenuates 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Mediated Induction of Myeloid-Derived Suppressor Cells (MDSC) and Their Functions.Nutrients. 2023 Nov 3;15(21):4667. doi: 10.3390/nu15214667. Nutrients. 2023. PMID: 37960320 Free PMC article.
References
-
- Abedi-Valugerdi M., Wolfsberger J., Pillai P. R., Zheng W., Sadeghi B., Zhao Y., et al. (2016). Suppressive effects of low-dose 5-fluorouracil, busulfan or treosulfan on the expansion of circulatory neutrophils and myeloid derived immunosuppressor cells in tumor-bearing mice. Int. Immunopharmacol. 40, 41–49. 10.1016/j.intimp.2016.08.023 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

