Balancing WNT signalling in early forebrain development: The role of LRP4 as a modulator of LRP6 function
- PMID: 37091972
- PMCID: PMC10119419
- DOI: 10.3389/fcell.2023.1173688
Balancing WNT signalling in early forebrain development: The role of LRP4 as a modulator of LRP6 function
Abstract
The specification of the forebrain relies on the precise regulation of WNT/ß-catenin signalling to support neuronal progenitor cell expansion, patterning, and morphogenesis. Imbalances in WNT signalling activity in the early neuroepithelium lead to congenital disorders, such as neural tube defects (NTDs). LDL receptor-related protein (LRP) family members, including the well-studied receptors LRP5 and LRP6, play critical roles in modulating WNT signalling capacity through tightly regulated interactions with their co-receptor Frizzled, WNT ligands, inhibitors and intracellular WNT pathway components. However, little is known about the function of LRP4 as a potential modulator of WNT signalling in the central nervous system. In this study, we investigated the role of LRP4 in the regulation of WNT signalling during early mouse forebrain development. Our results demonstrate that LRP4 can modulate LRP5- and LRP6-mediated WNT signalling in the developing forebrain prior to the onset of neurogenesis at embryonic stage 9.5 and is therefore essential for accurate neural tube morphogenesis. Specifically, LRP4 functions as a genetic modifier for impaired mitotic activity and forebrain hypoplasia, but not for NTDs in LRP6-deficient mutants. In vivo and in vitro data provide evidence that LRP4 is a key player in fine-tuning WNT signalling capacity and mitotic activity of mouse neuronal progenitors and of human retinal pigment epithelial (hTERT RPE-1) cells. Our data demonstrate the crucial roles of LRP4 and LRP6 in regulating WNT signalling and forebrain development and highlight the need to consider the interaction between different signalling pathways to understand the underlying mechanisms of disease. The findings have significant implications for our mechanistic understanding of how LRPs participate in controlling WNT signalling.
Keywords: LRP4; LRP6; WNT pathway; development; forebrain; genetic modifier; neuroepithelium; neuronal progenitor.
Copyright © 2023 Geng, Paul, Kowalczyk, Raimundo, Sporbert, Mamo and Hammes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

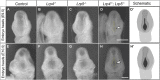
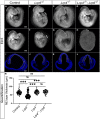
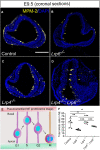
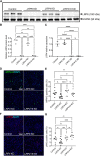

Similar articles
-
LRPs in WNT Signalling.Handb Exp Pharmacol. 2021;269:45-73. doi: 10.1007/164_2021_526. Handb Exp Pharmacol. 2021. PMID: 34490514 Review.
-
Lrp4 and Wise interplay controls the formation and patterning of mammary and other skin appendage placodes by modulating Wnt signaling.Development. 2013 Feb 1;140(3):583-93. doi: 10.1242/dev.085118. Development. 2013. PMID: 23293290 Free PMC article.
-
Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo.PLoS One. 2009 Nov 20;4(11):e7930. doi: 10.1371/journal.pone.0007930. PLoS One. 2009. PMID: 19936252 Free PMC article.
-
Competitive blocking of LRP4-sclerostin binding interface strongly promotes bone anabolic functions.Cell Mol Life Sci. 2022 Jan 31;79(2):113. doi: 10.1007/s00018-022-04127-2. Cell Mol Life Sci. 2022. PMID: 35099616 Free PMC article.
-
New Insights into Wnt-Lrp5/6-β-Catenin Signaling in Mechanotransduction.Front Endocrinol (Lausanne). 2015 Jan 20;5:246. doi: 10.3389/fendo.2014.00246. eCollection 2014. Front Endocrinol (Lausanne). 2015. PMID: 25653639 Free PMC article. Review.
Cited by
-
The Role of Autophagy in Copper-Induced Apoptosis and Developmental Neurotoxicity in SH-SY5Y Cells.Toxics. 2025 Jan 17;13(1):63. doi: 10.3390/toxics13010063. Toxics. 2025. PMID: 39853061 Free PMC article.
-
Epigenetic regulation by TET1 in gene-environmental interactions influencing susceptibility to congenital malformations.bioRxiv [Preprint]. 2024 Jul 8:2024.02.21.581196. doi: 10.1101/2024.02.21.581196. bioRxiv. 2024. PMID: 39026762 Free PMC article. Preprint.
-
LRP4 and Agrin Are Modulated by Cartilage Degeneration and Involved in β-Catenin Signaling in Human Articular Chondrocytes.Int J Mol Sci. 2025 Jan 24;26(3):1007. doi: 10.3390/ijms26031007. Int J Mol Sci. 2025. PMID: 39940775 Free PMC article.
-
Neuronal LRP4 directs the development, maturation and cytoskeletal organization of Drosophila peripheral synapses.Development. 2024 Jun 1;151(11):dev202517. doi: 10.1242/dev.202517. Epub 2024 Jun 3. Development. 2024. PMID: 38738619 Free PMC article.
-
Neuronal LRP4 directs the development, maturation, and cytoskeletal organization of peripheral synapses.bioRxiv [Preprint]. 2023 Nov 8:2023.11.03.564481. doi: 10.1101/2023.11.03.564481. bioRxiv. 2023. Update in: Development. 2024 Jun 1;151(11):dev202517. doi: 10.1242/dev.202517. PMID: 37961323 Free PMC article. Updated. Preprint.
References
-
- Allache R., Lachance S., Guyot M. C., De Marco P., Merello E., Justice M. J., et al. (2014). Novel mutations in Lrp6 orthologs in mouse and human neural tube defects affect a highly dosage-sensitive Wnt non-canonical planar cell polarity pathway. Hum. Mol. Genet. 23, 1687–1699. 10.1093/hmg/ddt558 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

